CHADS2 ≥1 Or - Programme Fa

F A - C I L I T E R — C o o r d i n a t o r W o r k P l a n
D a t e : M a y 2 3 , 2 0 1 2
(FA-CILITER PROGRAM — Atrial Fibrillation in an Integrated Clinic to Limit Thromboembolic Events)
C o o r r d i i n a t t o r r W o r r k P l l a n
Is the patient medically stable AND available for 1 hour of assessment and counseling?
Yes No
Give the patient the FA-CILITER Program pamphlet and ask the patient if he/she agrees to participate in the
FA-CILITER Program and proceed with Visit 1, if possible.
Visit 1
Give the patient the FA-CILITER Program summary and record the patient’s contact information for future follow-up.
□ Complete the demographic sheet.
□ Complete the data sheet (Visit 1).
□ Ask the patient to complete the FA-CILITER Quality of Life Questionnaire.
□ Obtain resting ECG.
□ Obtain laboratory test values (hemoglobin, platelet count, glycemia , serum creatinine, creatinine clearance
1
, glomerular filtration rate
2
, ejection fraction, echocardiogram, thyroid function and electrolytes).
□ Calculate CCS-SAF score.
□ Calculate CHADS
2
and CHADS
2
-VASC score.
□ Calculate HAS-BLED score.
□ Determine whether there are any reversible causes of atrial fibrillation.
□ Counseling patient on AF management, patient educational material, website, visit card.
□ Schedule date for next telephone call.
□ Send an information letter to the patient’s physician(s) and pharmacist(s) concerning the patient’s participation in the FA-CILITER program.
1
Creatinine clearance: (140-age) x weight (kg) x constant (1.23 for males, 1.04 for females)/serum creatinine
(µmol/L).
2
MDRD formula: 186 x (creatinine/88.4)
-1.154
x (Age)
-0.203
x (0.742 if female) x (1.210 if Black African) or glomerular filtration rate estimated by the local laboratory.
C ongestive heart failure
H ypertension
A ge ≥ 75 years
D iabetes mellitus
Prior S troke or TIA
Score
CHADS
2
Score
May 23, 2012
1
1
2
1
1
_____ /6
Page 2
C ongestive heart failure
CHADS
2
-VASC Score
1
May 23, 2012
H ypertension
D iabetes mellitus
Prior S troke or TIA
A ge ≥ 75 years
A ge ≥ 65 years
Female S ex
V ascular disease (peripheral or coronary artery disease)
SCORE
2
1
1
1
_____ /9
1
1
2
Page 3
May 23, 2012
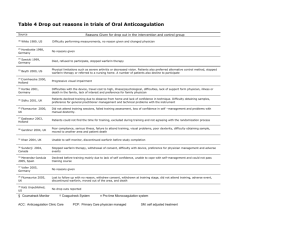
Absence of coronary artery disease or stable coronary artery disease
□
CHADS
2
≥1
Or
□
CHADS
2
= 0 with age ≥65 years or woman with peripheral vascular disease
□
ANTICOAGULANT
3
□
If the patient is not anticoagulated, inform the
FA-CILITER physician
For patients on warfarin
3 Recommendations CCC 2012: Novel oral anticoagulant is preferable to warfarin.
Page 4
May 23, 2012
INR therapeutic and stable?
□ If yes, maintain on warfarin
□ If not, consider change warfarin to dabigatran
If use dabigatran
Inform the FA-CILITER physician of creatinine clearance and glomerular filtration
□ Normal renal function : dabigatran à 150 mg bid
□ Abnormal renal function
4
,
5
:
□ If ClCr ≤30 ml/min, change to warfarine
□ If ClCr de 31-60 ml/min, consider dabigatran at 110 mgbid
□ If patient is older than >80 years, dabigatran at 110 mgbid
Other anticoagulant
Note reason for use of other anticoagulant
□ CHADS
2
= 0 and woman or peripheral vascular disease : consider Aspirin
□ For patients with GFR <15 ml/min and on dialysis, CCC 2012 do not recommend routine use of oral anticoagulants or ASA in these patients.
4
The above suggestion of dose for dabigatran is based on the product monography et the RE-LY study. Of note, creatine clearance can over-estimate the GFR (as measured by MDRD) by 10-20%. To inform the FA-
CILITER physician (creatinine clearance and glomerular filtration).
5
Canadian Cardiovascular Society 2012 recommends use of GFR to evaluate kidney function. Dabigatran
150 bid ou 110 bid can be used for patients with GFR 30-59 ml/min
Page 5
May 23, 2012
Recent Acute Coronary Syndrome or
Percutaneous Coronary Intervention
CHADS
2
≤1 CHADS
2
≥2
ASA & clopidogrel ASA & clopidogrel & anticoagulant
6
If CHADS
2
≥2 and not anticoagulated, inform the FA-CILITER physician
6 Recommendations CCC 2012 : Warfarin is preferred over novel oral anticoagulant
Page 6
For patients on warfarin
INR therapeutic and stable?
May 23, 2012
□ If yes, maintain on warfarin
□ If not, consider change warfarin to dabigatran
If use dabigatran
Inform the FA-CILITER physician of creatinine clearance and glomerular filtration
□ Normal renal function : dabigatran à 150 mg bid
□ Abnormal renal function
7
,
8
:
□ If ClCr ≤30 ml/min, change to warfarine
□ If ClCr de 31-60 ml/min, consider dabigatran at 110 mgbid
□ If patient i solder than >80 years, dabigatran at 110 mgbid
Other anticoagulant
Note reason for use of other anticoagulant
□ CHADS
2
= 0 and woman or peripheral vascular disease : consider Aspirin
□ For patients with GFR <15 ml/min and on dialysis, CCC 2012 do not recommend routine use of oral anticoagulants or ASA in these patients.
7
The above suggestion of dose for dabigatran is based on the product monography et the RE-LY study. Of note, creatine clearance can over-estimate the GFR (as measured by MDRD) by 10-20%. To inform the FA-
CILITER physician (creatinine clearance and glomerular filtration).
8
Canadian Cardiovascular Society 2012 recommends use of GFR to evaluate kidney function. Dabigatran
150 bid ou 110 bid can be used for patients with GFR 30-59 ml/min
Page 7
May 23, 2012
HAS-BLED Score
Hypertension (>160 mmHg systolic)?
Yes +1
Kidney disease (dialysis, serum creatinine >200 µmol/L)?
Liver disease (cirrhosis, bilirubin >2x normal limit,
AST/ALT/AP >3x normal limit)?
Prior stroke or TIA?
Yes +1
Yes +1
Yes +1
Prior major bleeding or predisposition to bleeding?
Yes +1
Labile INR (INR <60% of time in the therapeutic range)?
Yes +1
Age ≥65 years?
Drugs that increase bleeding risk (anti-platelet agents, nonsteroidal anti-inflammatory drugs)?
Excessive use of alcohol?
Yes +1
Yes +1
Yes +1
SCORE
_________/9
□ If HAS-BLED score > CHADS
2
score
OR
□ HAS-BLED score ≥3
□ Notify FA-CILITER physician
(The risk of bleeding may outweigh the benefit of anticoagulation therapy.)
Page 8
May 23, 2012
Identifying the Reversible Causes of Atrial Fibrillation
Determine whether any of these underlying conditions may have triggered or worsened the AF:
_____Hypertension
_____Hyperthyroidism
_____Consumption of alcohol (provide alcohol cessation advice)
_____Consumption of drugs or stimulants (e.g., caffeine, medications for the relief of flu-like illnesses)
_____Acute coronary syndrome
_____Cardiac surgery
_____Non-cardiac surgery
_____Acute respiratory disorder
_____Infection
_____Other (specify) ______________________________________________
If any of these conditions are present, are not being treated or remain uncontrolled, notify the FA-CILITER physician and/or family physician.
Page 9
May 23, 2012
Telephone Call at Month 1, Month 3 and Month 6
□ Complete sheets for Month 1, 3 and 6 visits.
□ Review treatment compliance. o Encourage compliance. o Determine whether there has been a change in treatment.
□ Answer the patient’s questions.
□ If the patient cannot be reached, call the family or another contact person.
□ Complete and forward the follow-up letter to the family physician and pharmacist, if applicable.
□ Recalculate CHADS
2
, CHADS
2
-VASC and HAS-BLED scores.
□ If these scores have changed, review anticoagulation therapy with the FA-CILITER physician.
Final Visit at Month 12
□ Complete final visit data sheet.
□ Review medications.
□ Review treatment compliance. o Encourage compliance. o Determine whether there has been a change in treatment.
□ Answer the patient’s questions.
□ Provide patient with advice on future care.
□ Obtain resting ECG.
□ Recalculate CHADS
2
, CHADS
2
-VASC and HAS-BLED scores.
□ If these scores have changed, review anticoagulation therapy with the FA-CILITER physician.
□ Re-determine patient’s renal function.
□ Send a letter to the cardiologist, family physician and pharmacist, explaining that the
FA-CILITER Program has been completed.
□ Complete the Quality of Life and CCS-SAF questionnaires.
Page 10
Stroke
May 23, 2012
□ Notify the FA-CILITER physician and/or cardiologist and/or patient’s family physician (if applicable).
Major bleeding (requiring a transfusion, emergency room visit or hospitalization, or resulting in a change in medication or death)
□ Notify the FA-CILITER physician and/or cardiologist and/or patient’s family physician (if applicable).
Unscheduled patient call(s) or visit(s)
□ Record reason and intervention performed.
□ Notify the FA-CILITER physician and/or cardiologist and/or patient’s family physician.
Patient death
□ Obtain reason and date of death from family.
□ Obtain death certificate (if possible).
□ Notify the FA-CILITER physician.
Page 11
F A - C I L I T E R — C o o r d i n a t o r W o r k P l a n
D a t e : M a y 2 3 , 2 0 1 2
Quality of Life Questionnaire
(To be completed by the patient)
Date: ____________ (day/month/year)
□ At the initiation of the FA-CILITER Program
□ At the completion of the FA-CILITER Program
Please rate your current health status on the following scale.
Worst health status Best health status
Please rate your current state of well-being on the following scale.
Worst feeling of well-being Best feeling of well-being
May 23, 2012
Work*
Have your AF symptoms affected your work?
Not at all Mildly Moderately Somewhat Severely
Has your treatment for AF affected your work?
Not at all Mildly Moderately Somewhat Severely
I have not worked lately due to reasons unrelated to AF.
*: Work includes remunerative work, volunteer work or training.
Social Life
Have your AF symptoms affected your social life/leisure activities?
Not at all Mildly Moderately Somewhat Severely
Has your treatment for AF affected your social life and/or your leisure activities?
Not at all Mildly Moderately Somewhat Severely
Family Life and Household Responsibilities
Have your AF symptoms affected your family life and household responsibilities?
Not at all Mildly Moderately Somewhat Severely
Has your treatment for AF affected your family life and household responsibilities?
Not at all Mildly Moderately Somewhat Severely
Page 13
May 23, 2012
Missed days of work (approximate estimate)
How many days of work have you missed in the last 6 months or how many days have you not been able to carry out your normal responsibilities due to your AF symptoms?
Productivity (approximate estimate)
How many days in the last 6 months did you have reduced productivity due to your AF symptoms?
Page 14
CCS-SAF Scale
May 23, 2012
The Three Steps
•
Step 1 – Symptoms
•
Palpitation
•
Dyspnea
•
Dizziness, pre-syncope or syncope
•
Chest pain
•
Weakness or fatigue
•
Step 2 – Association
•
Are these symptoms present during an AF episode?
•
Determine if these symptoms are associated with an AF episode (or if they are due to another cause).
•
Step 3 – Functionality
•
Determine if the AF symptoms (or their treatment) affect the patient’s functionality
(subjective quality of life).
•
Give a score of 0-4 based on the criteria listed in the table.
Page 15
May 23, 2012
Table 1: Canadian Cardiovascular Society Severity of Atrial Fibrillation Scale
SAF Class Effect on patient’s general quality of life (QoL)
0 Asymptomatic with regard to AF
1
2
Symptoms attributable to AF have a minimal effect on patient’s general QoL
Minimal and/or rare symptoms, or
Single AF episode without syncope or heart failure
Symptoms attributable to AF have a minor effect on patient’s general QoL
Mild cognizance of symptoms in patients with persistent/permanent AF, or
Rare episodes (e.g., less than a few per year) in patients with paroxysmal or intermittent AF
3
4
Symptoms attributable to AF have a moderate effect on patient’s general QoL
Moderate cognizance of symptoms most days in patients with persistent/permanent AF, or
More frequent episodes (e.g., more than every few months) or more severe symptoms, or both, in patients with paroxysmal or intermittent AF
Symptoms attributable to AF have a severe effect on patient’s general QoL
Very unpleasant symptoms in patients with persistent/paroxysmal AF and/or
Frequent and highly symptomatic episodes in patients with paroxysmal or intermittent AF and/or
Syncope believed to be caused from AF and/or
Heart failure secondary to AF
Page 16
May 23, 2012
APPENDIX — Example of Letter to Family Physician – First Visit
(Name of city) _______
(Date)__________________
SUBJECT: Optimization of your patient’s medical treatment for atria fibrillation
Dr. __________________ (treating physician),
Mr./Ms._______________________ (pharmacist)
Your patient, Mr./Ms. _________________________ is currently taking part in [name of institution]’s FA-CILITER (Atrial Fibrillation in an Integrated Clinic to Limit Thromboembolic
Events) Program.
FA-CILITER is a multidisciplinary care program whose mission is to optimize care, follow-up and education to prevent thromboembolic complications in patients with atrial fibrillation. This program does not include INR monitoring follow-up.
We would like to share our opinion about your patient’s anticoagulation therapy with you:
This patient has a low risk of stroke and does not require an anticoagulant.
This patient has a high risk of stroke and we have initiated anticoagulation therapy with
____________________.
This patient was taking warfarin; however, given that:
His/her INR levels are labile, we have switched the warfarin to dabigatran.
INR monitoring is difficult, we have switched the warfarin to dabigatran.
Due to _____________________________________________________, we have switched the warfarin to dabigatran ________mg BID.
This patient was taking dabigatran; however, due to his/her renal disease, we have switched the dabigatran to warfarin.
Page 17
May 23, 2012
This patient was on anticoagulation therapy; however, given his/her high risk of bleeding due to __________________________________, we have discontinued the anticoagulation therapy.
We have initiated ASA at a dose of __________________ per day.
We also suggest the continuation of treatment with these medications:
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
We suggest the discontinuation of these medications:
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
We suggest making changes to these medications:
______________________________________________________________________________
______________________________________________________________________________
Other suggestions:
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
Enclosed you will find information that may help you with the follow-up of your patient:
□ Hospitalization summary or ER visit note
□ Copy of anticoagulant prescription
□ FA-CILITER pamphlet
□ FA-CILITER visit card
Page 18
May 23, 2012
If you have any questions about the FA-CILITER Program and/or information about your patient, please contact Mr./Ms. _________________________ (FA-CILITER Program Coordinator) at
______________________________________________.
We thank you for your cooperation and the attention afforded this letter.
Sincerely,
____________________________________
Physician
____________________________________
Coordinator
Page 19
May 23, 2012
APPENDIX — Example of FA-CILITER Program Follow-up Letter
(Name of city) _______
(Date)__________________
SUBJECT: Your patient’s telephone follow-up call at _____ months
Dr. __________________ (treating physician),
Mr./Ms. ________________________ (pharmacist)
Your patient, Mr./Ms. _________________________, is a participant in the [name of institution]’s FA-CILITER (Atrial Fibrillation in an Integrated Clinic to Limit Thromboembolic
Events) Program.
FA-CILITER is a multidisciplinary care program whose mission is to optimize care, follow-up and education to prevent thromboembolic complications in patients with atrial fibrillation. This program does not include INR monitoring follow-up.
As part of the FA-CILITER Program, we wish to share your patient’s health status with you.
He/she is asymptomatic.
He/she suffers from class _______ angina.
He/she suffers from class _______ dyspnea.
He/she suffers from class ______ palpitations.
He/she visited the ER on _____________ (date) for ____________________ (reason).
He/she was hospitalized from ___________ to _____________ because
_____________________________.
He/she suffered a transient ischemic attack (TIA) on ____________________________.
He/she suffered a stroke on _______________________.
He/she experienced ________________ bleeding.
Page 20
May 23, 2012
Development of new diseases or a new lifestyle since the last FA-CILITER follow-up
High blood pressure
Heart failure
Diabetes mellitus
Coronary artery disease
Peripheral artery disease
Liver disease
Excessive consumption of alcohol
Regular administration of anti-inflammatory drugs
Regular administration of anti-platelet agents
Medical Treatment
□ He/she is not receiving anticoagulation therapy because ________________.
□ Optimal anticoagulation is being achieved with ______________.
□ His/her anticoagulation therapy has been discontinued because _______________.
We suggest the continuation of treatment with these medications:
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
We suggest the discontinuation of these medications:
______________________________________________________________________________
______________________________________________________________________________
We suggest making changes to these medications:
______________________________________________________________________________
______________________________________________________________________________
Page 21
May 23, 2012
Other suggestions:
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
Enclosed you will find information that may help you with the follow-up of your patient:
□ Hospitalization summary or ER visit note
□ Other document ____________________
If you have questions about the FA-CILITER Program and/or information about your patient, please contact Mr./Ms. __________________________ (FA-CILITER Program Coordinator) at
______________________________________________.
Thank you for your cooperation and the attention afforded this letter.
Sincerely,
____________________________________
Physician
____________________________________
Coordinator
Page 22
May 23, 2012
APPENDIX —Example of FA-CILITER Program Completion Letter
(Name of city) _______
(Date)__________________
SUBJECT: Your patient’s final visit at _____ Months
Dr. __________________ (treating physician),
Mr./Ms. ________________________ (pharmacist)
Your patient, Mr./Ms. _________________________, is a participant in the [name of institution]’s FA-CILITER (Atrial Fibrillation in an Integrated Clinic to Limit Thromboembolic
Events) Program.
We would like to inform you that your patient has completed the FA-CILITER Program.
Symptoms
He/she is asymptomatic.
He/she suffers from class _______ angina.
He/she suffers from class _______ dyspnea.
He/she suffers from class ______ palpitations.
He/she visited the ER on _____________ (date) for ____________________ (reason).
He/she was hospitalized from ___________ to _____________ because
_____________________________.
He/she suffered a transient ischemic attack (TIA) on ____________________________.
He/she suffered a stroke on _______________________.
He/she experienced ________________ bleeding.
Page 23
May 23, 2012
Development of new diseases or a new lifestyle since the last FA-CILITER follow-up
High blood pressure
Heart failure
Diabetes mellitus
Coronary artery disease
Peripheral artery disease
Liver disease
Excessive consumption of alcohol
Regular administration of anti-inflammatories
Regular administration of anti-platelet agents
Medical Treatment
□ He/she is not receiving anticoagulation therapy because ________________.
□ Optimal anticoagulation is being achieved with ______________.
□ His/her anticoagulation therapy has been discontinued because _______________
We suggest the continuation of treatment with these medications:
______________________________________________________________________________
We suggest discontinuation of these medications:
______________________________________________________________________________
______________________________________________________________________________
We suggest making changes to these medications:
______________________________________________________________________________
______________________________________________________________________________
Other suggestions:
______________________________________________________________________________
______________________________________________________________________________
Page 24
May 23, 2012
Enclosed you will find information that may help you with the follow-up of your patient:
□ Hospitalization summary or ER visit note
□ Other document________________________________
It was our pleasure to care for the patient during the past year. We hope that the patient has benefited from our advice and services. We have asked him/her to continue his/her medical follow-up with you.
If you have questions about the FA-CILITER Program and/or information about your patient, please contact Mr./Ms. __________________________ (FA-CILITER Program Coordinator) at
______________________________________________.
Thank you for your cooperation and the attention afforded this letter.
Sincerely,
____________________________________
Physician
____________________________________
Coordinator
Page 25