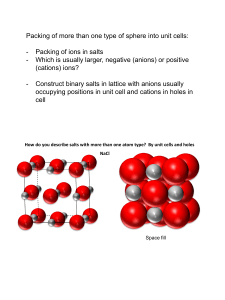
Quick Answers, to…. IN- AND OUT-OF
advertisement

Created by Joanne L. Stewart, Department of Chemistry, Hope College (stewart@hope.edu) and posted on www.ionicviper.org April 2, 2008. Copyright Joanne L. Stewart, 2008. This work is licensed under the Creative Commons Attribution Noncommercial Share Alike License. To view a copy of this license visit creativecommons.org/licenses/by-nc-sa/3.0/. Quick Answers, to…. IN- AND OUT-OF-CLASS EXERCISE CHEM 341 http://firstyear.chem.usyd.edu.au/calculators/solid_state.shtml?tab=2 JMOL MODELLING EXERCISE STEP 1. BUILD THE MODELS FIRST. STEP 2. WHEN ANSWERING QUESTIONS, MAKE SURE EVERYONE IN YOUR GROUP “SEES” IT. STRUCTURES OF IONIC SOLIDS A. Filling all the octahedral holes: NaCl and NiAs Which one of these structures has hcp anions? Which one has ccp anions? Remember that you can view a solid lattice as being comprised of close packed anions, with the cations fitting into small ‘holes’ in this lattice. For example: NaCl. The ‘close packed arrangement’ of Clions displays the layered view, where you can see the ABC pattern of anions in this CCP arrangement. You might also be able to see that this lattice can be viewed as a cubic lattice with four diagonal layers (ABCA). So it looks like “CCP” gets its name from this cubic view. Similarly, NiAs can be viewed as a close-packed lattice of As2- anions with Ni2+fitting into gaps (“holes”) within the lattice. The ABABAB patterns of the As2=ions cannot be simplified into a cubic lattice, so the unit cell has to be hexagonal HCP. What is the coordination environment (coordination number and geometry) about the anions in NaCl? What is the coordination environment about the cations in NaCl? What is the coordination environment about the anions in NiAs? What is the coordination environment about the cations in NiAs? What is the stoichiometry of the NiAs unit cell? Explain. What is the stoichiometry of the NaCl unit cell? Explain. Stoichiometry means the # and ratio of ions in the lattice. So count carefully, and remember your chemical intuition. You know that the ratio of ions for each lattice MUST BE 1:1 DUE TO CHARGE BALANCE (no, the caps was not stuck). But you have to count each fractional ion contained within the unit cell (which has to repeat infinitely) – so NaCl has 4 Na+ and 4 Cl- ions. NiAs is definitely more challenging, as it has a hexagonal unit cell and the JMOL animation included two As2- from adjacent unit cells within the middle layer. Read pg. 230 of your textbook, as this will help (but note that the book shows Ni2+ layers, while the JMOL shows As2- layers)! In the book, you can see that the unit cell has a top layer and a bottom layer of 6 edge Ni2+ (each worth 1/6 per unit cell) and one face-centered Ni2+ (worth per unit cell); the middle layer has 6 edge Ni2+ (worth 1/3) and a body-centered Ni2+ (worth 1). So the total # Ni2+ = [6(1/6)+1/2] + [6(1/3) + 1] + [6(1/6) + )] =six. Created by Joanne L. Stewart, Department of Chemistry, Hope College (stewart@hope.edu) and posted on www.ionicviper.org April 2, 2008. Copyright Joanne L. Stewart, 2008. This work is licensed under the Creative Commons Attribution Noncommercial Share Alike License. To view a copy of this license visit creativecommons.org/licenses/by-nc-sa/3.0/. The total # As2- = six (all within the unit cell), And a big sigh of relief that the Ni/As ratio is 6/6 = 1/1. lattice is essentially an ABA layered structure of As2- with Ni2+in between the layers. B. Filling half of the octahedral holes: cadmium chloride and cadmium iodide Which one has hcp anions and which one has ccp anions? What is the coordination environment of the cadmium ion in each of these two structures? What are the stoichiometries of the unit cells for each of these structures? Explain. Finding the holes is the trick here. I suggest that you view each structure as a close-packed arrangement of anions, then observe how cations are found within the holes. Page 226 of your text has a helpful figure. Also, each parent lattice example (top of the JMOL page) has buttons to show you the T+ and T- holes, and the octahedral holes. CdI has the ‘layered’ view because of stoichiometry: only half of the available octahedral holes can be occupied to keep the Cd2+:I- ratio at 1:2. There is one octahedral hole per anion, so if all holes were filled the ratio would be 1:1 (and we’d have too many positive charges). C. Filling half of the tetrahedral holes: zinc blende and wurtzite What is the coordination environment of each of the ions in the zinc blende structure? What is the stoichiometry of the zinc blende unit cell? Explain. What is the coordination environment of each of the ions in wurtzite? What is the stoichiometry of the wurtzite unit cell? Explain. Zinc blende has easily observed tetrahedral holes. Since each anion creates two tetrahedral holes in a close packed lattice, only half of the tetrahedral holes are occupied. Be sure to find the ABC layers of the CCP lattice (zinc blende); and find the AB layers of the hcp lattice (sphalerite). D. Filling all of the tetrahedral holes: anti-fluorite and fluorite What is the coordination environment of each of the ions in the anti-fluorite structure? What is the coordination environment of each of the ions in the fluorite structure? What is the stoichiometry of the anti-fluorite unit cell? Explain. What is the stoichiometry of the fluorite unit cell? Explain. Explain why these two structures are named so similarly? What is the relationship?