AP Biology Chapter 54 notes Ecosystems Ecosystem ecology
advertisement

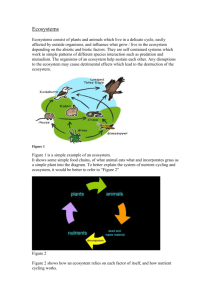
AP Biology Chapter 54 notes Ecosystems Ecosystem ecology emphasizes energy flow and chemical recycling An ecosystem consists of all the organisms in a community and all the abiotic factors with which they interact The laws of physics and chemistry apply, especially energy flow Energy is conserved but degraded to heat during the ecosystem processes Energy and nutrients pass from primary producers (autotrophs) to primary consumers (herbivores) to secondary consumers (carnivores). Energy flows through an ecosystem, entering as light and exiting as heat. Nutrients cycle within the ecosystem. Decomposition connects all trophic levels. Detritivores, mainly bacteria and fungi recycle essential chemical elements by decomposing organic matter and returning the elements to inorganic reservoirs ( soil, water, air) Ecosystem Energy budgets: The energy produced through photosynthesis is a tiny fraction of the solar radiation reaching the Earth, but primary production sets the spending limit for the global energy budget. Most primary producers use light energy to synthesize energy rich organic molecules, which can be broken down to generate ATP. Consumers acquire their organic fuels through the food web (2nd hand, 3rd hand etc). So, the extent of the photosynthetic production sets the spending limit for energy budget of the entire ecosystem Only a small fraction (1%) of the visible light that does reach the photosynthetic organisms is converted to chemical energy. But collectively, the primary producers on Earth create about 170 billion tons of organic material/year. Gross primary production is the total energy assimilated by an ecosystem in a given time period. (the amount of light energy converted to chemical energy by photosynthesis per unit time). The net primary production, the energy accumulated in the autotroph biomass, equals the gross primary production minus the energy used by the primary producers for respiration. Only net primary production is available to consumers. NPP=GPP-R Ecologists use net primary production because it’s a key measurement for the storage of chemical energy that will be available to consumers in the ecosystem. Net primary production can be expressed as energy per unit area per unit time (J/m2/yr) or as biomass (weight) of vegetation added to the ecosystem per unit area per unit time (g/m2/yr). Biomass is usually expressed in dry weight because the water content in plants varies greatly over short periods of time. In Marine and freshwater ecosystems: Light and nutrients limit primary production. Within the photic zone the factor that most often limits primary production is a nutrient such as nitrogen or iron. A limiting nutrient is the element that must be added to in order for production to increase in a particular area. The nutrients most often limiting marine production is either nitrogen or phosphorus. Concentrations of these nutrients are very low in the photic zone where phytoplankton live. But they are abundant is deep water where there is not enough light for photosynthesis. In areas of up-welling (where nutrient rich deep water is circulated to the surface) the steady supply of nutrients stimulates growth of phytoplankton populations that form the basis of marine food webs. Nutrient limitation is also prevalent in fresh water lakes. The blooms of cyanobacteria (eutrophication) are caused by the runoff of fertilizers and sewage from farms and yards In terrestrial or wetlands ecosystems: Climate factors such as temperature and moisture affect primary production on a large geographic scale. More locally, soil nutrients are often the limiting factor in primary production. Nitrogen and phosphorus are most often the limiting nutrients limiting terrestrial and wetland production. Production efficiency: The amount of energy available to each trophic level is determined by the net primary production and the efficiencies in which food energy is converted to biomass at each link in the food chain. The amount of chemical energy in consumers’ food that is converted to their own new biomass during a given time period is called secondary production of the ecosystem. Trophic efficiency: is the percentage of energy transferred from one trophic level to the next – is generally 5-20% depending on the type of ecosystem. The loss of energy with each transfer in a food chain can be represented by a pyramid of net production. (see figure 54.11 pg 1192) Pyramid of Biomass: each tier represents the standing crop in one trophic level. Pyramids of numbers: the progressive loss of energy along the food chain severely limits the overall biomass of top-level consumers that an ecosystem can support. This helps explain why food webs include only about 4-5 trophic levels. Green World Hypothesis: herbivores consume a small percentage of vegetation because predators, disease, competition, nutrient limitations, and other factors keep their populations in check. Several factors that keep herbivores in check: 1. Plants have defenses against herbivores (spines, noxious chemicals) 2. Essential nutrients, not energy supply usually limit herbivores 3. Abiotic factors (temp, moisture) 4. Intraspecific competition (territorial behavior) 5. Interspecific interactions keep herbivore density in check Biogeochemical cycle: Atoms present in the complex molecules of an organism when it dies are returned to the atmosphere, water, or soil by the action of decomposers. This decomposition replenishes the pool of inorganic nutrients that plants and other autotrophs use to build organic matter. These nutrient circuits involve both biotic and abiotic components. A General Model of Chemical Cycling: Gaseous forms of carbon, oxygen, nitrogen, and sulfur in the atmosphere cycle globally. Other, less mobile nutrients, including, phosphorus, potassium, and calcium cycle on a more localized scale, at least over the short term. All elements cycle between organic and inorganic reservoirs. Ecologists study chemical cycling by adding tiny amounts of radioactive isotopes of the elements they want to track or by following the movement of naturally occurring stable, non-radioactive isotopes through the various biotic and abiotic components of an ecosystem. Biochemical cycles: Water moves in a global cycle driven by solar energy. The carbon cycle primarily reflects the reciprocal processes of photosynthesis and cellular respiration. Nitrogen enters the ecosystems through atmospheric deposition and nitrogen fixation by prokaryotes, but most of the nitrogen cycling in natural ecosystems involves local cycles between organisms and soil and water. The phosphorus cycle is relatively localized through the weathering of rocks that adds phosphate to the soil; with some leaching into the ground water and surface water. The decomposition and nutrients: The rates at which the nutrients cycle in different ecosystems are extremely variable, mostly as a result of the rates of decomposition. Temperature and availability of water affect the rates of decomposition and thus nutrient cycling times. The rate of decomposition in terrestrial ecosystems increases with the actual evapotranspiration (annual amount of water transpired by plants and evaporated from a landscape). Other factors influencing nutrient cycling, soil chemistry and frequency of fires. Human population is disrupting the trophic structure, energy flow and chemical cycling of ecosystems in most areas of the world. Nutrient enrichment: Example: nutrients in farm soil may runoff into streams and lakes, resulting in nutrient depletion in one area, excesses in another, and the disruption of the natural chemical cycles in both locations. Nitrogen is the main nutrient lost through agriculture, having a large impact on the nitrogen cycle. Ex. farmers use fertilizers to replenish the nitrogen in their fields. Recent studies indicate that human activities have doubled the amount of fixed nitrogen available to the primary producers. The key problem with excess nitrogen seems to be critical load, which is the amount of nutrient that can be absorbed by the plants without damaging the ecosystem integrity. Excess nitrogen eventually leaches into the groundwater or run off directly into fresh water and marine ecosystems contaminating water supplies, choking waterways, and killing fish. Acid Precipitation: The burning of wood, coal and other fossil fuels releases oxides of sulfur and nitrogen that react with the water in the atmosphere, forming sulfuric and nitric acid. The acids fall to Earth and generally have a pH level less than 5.6. This lowers the pH levels of aquatic environs and the affects the soil chemistry of terrestrial ecosystems. Toxins in the environment: humans release a large variety of toxic chemicals with little regard to ecological consequences Biological magnification: Toxins become increasingly more concentrated in successive trophic levels of the food web. Top level predators tend to be the organisms that are most affected. Atmospheric CO2 has been steadily increasing. The ultimate effects may include significant warming and other climate change. The Ozone layer reduces the penetration of UV radiation through the atmosphere. Human activities including the release of chlorine containing pollutants are eroding the ozone layer.