Growth of the Two Layers of the Chick Sclera Is Modulated
advertisement

Growth of the Two Layers of the Chick Sclera Is
Modulated Reciprocally by Visual Conditions
Daniel Marzani and Josh Wallman
Purpose. Although visual deprivation causes increased ocular elongation and myopia in both
birds and mammals, changes in sclera appear to be in opposite directions. Because avian
sclera has a cartilaginous layer as well as the fibrous layer found in mammals, we examined
whether the scleral responses to various visual manipulations differ between the two layers.
Methods. To produce increases in ocular elongation and myopia, monocular diffusers or
negative lenses were fitted to eyes. Conversely, to produce decreases in ocular elongation,
diffusers were removed (restoring normal vision) or monocular positive lenses were fitted.
Scleral layers were then dissected apart, and incorporation of labeled precursors into glycosaminoglycans (GAGs), DNA, and protein was assessed. Tissue coculture experiments were
used to assess humoral interactions between scleral layers and with the choroid.
Results. In the cartilaginous layers, the incorporation of label into proteoglycans and DNA
was significantly higher in eyes elongating faster than normal because of wearing diffusers or
negative lenses and significantly lower than normal in eyes elongating slower than normal
because of removal of the diffuser or wearing positive lenses. In the fibrous layers, the reverse
was the case. Coculturing cartilaginous sclera from normal eyes with fibrous sclera from
myopic or recovering eyes produced the same increase or decrease in sulfate incorporation
into GAGs in the cartilaginous layer as though the tissue measured was from the animal
providing the conditioning tissue. Coculturing with choroid, especially from recovering eyes,
also inhibited cartilaginous sclera.
Conclusions. The fibrous layer of the avian sclera shows changes in sulfate incorporation into
GAGs during deprivation and recovery from deprivation in the same direction as does the
mammalian sclera, whereas the cartilaginous layer changes in the opposite direction. The
responses of the cartilaginous layer may be controlled by the fibrous layer, although they are
influenced by the choroid as well. Invest Ophthalmol Vis Sci. 1997;38:1726-1739.
JL owerful evidence that visual stimulation affects eye
growth comes from the fact that in many species of
bird and mammal, visual form deprivation by means
of diffusers or closed lids causes increased ocular elongation and myopia.1"7 Although the phenomenon of
form-deprivation myopia seems similar in birds and
mammals, the scleral changes seem to be in opposite
directions.
In birds, the increased ocular elongation caused
by form deprivation is associated with increased
From the Department of Biology, City College of the City University of New York,
Neiu York, Neiu York..
Supported by National Institutes of Health grant R37EY02727.
Submitted for publication July 15 1996; revised March 17, 1997; accepted March
18, 1997.
Proprietary interest calegoiy: N.
Reprint requests: Daniel Marzani, Department of Biology, City College of the City
University of Neiu York, Neiu York, NY 10031.
1726
growth of the sclera, as shown by increased dry weight
and increased incorporation of precursors into DNA,
protein, and glycosaminoglycans (GAGs).8'9 Conversely, if a visually deprived eye has form vision restored, the eye slows its rate of elongation below normal until the length appropriate for its age and focal
length is attained. 1011 Correspondingly, during this
recovery period, incorporation of precursors into
GAGs, protein, and DNA is reduced below normal
levels.8'12'13
In mammals, in contrast, visual deprivation is associated with decreased scleral dry weight, decreased
proteoglycan content, and decreased rate of incorporation of precursors into GAGs.1415 Conversely, eyes
recovering from visual deprivation show increased incorporation of precursors into GAGs, but no change
in DNA or GAG levels, relative to nondeprived eyes.16
Investigative Ophthalmology & Visual Science, August 1997, Vol. 38, No. 9
Copyright © Association for Research in Vision and Ophthalmology
1727
Vision Modulates Growth of Chick Scleral Layers
CH
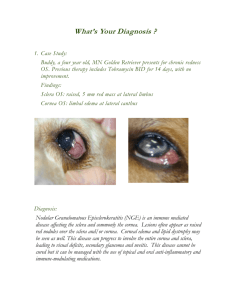
FIGURE 1. Light microscopic
photograph of section of
posterior wall of normal
chick eye (14 days old)
stained with hematoxylineosin, showing the two layers of sclera. FS = fibrous
sclera; CS = cartilaginous
sclera; CH = choroid. Scale
bar = 50 /xm.
We suggest that the mechanism of scleral remodeling is similar in birds and mammals in these experimental situations, and that the apparent difference
arises from the fact that the chick sclera is composed
of two layers: an outer fibrous layer (like that in mammals), which contains collagen type I and small proteoglycans such as decorin, and an inner cartilaginous
layer, which contains collagen types II and IV and
aggrecan as the predominant proteoglycan8 (Fig. 1).
In the experiments presented here, we separated the
two layers and found that the fibrous layer resembles
the mammalian sclera in diat it decreases incorporation of precursors into GAGs and DNA as the eye
elongates and becomes myopic during visual deprivation, whereas the cartilaginous layer increases incorporation into GAGs and DNA under these conditions.
Conversely, when the rate of ocular elongation is reduced below normal by giving deprived eyes normal
visual conditions, incorporation into GAGs decreases
in the cartilaginous layer and increases in the fibrous
layer.
The rate of elongation of avian and mammalian
eyes can also be increased or decreased by imposing
hyperopic or myopic defocus by negative or positive
spectacle lenses, respectively. In chicks, wearing lenses
causes opposite changes in sulfate incorporation into
scleral GAGs.17 We show that the two scleral layers
show opposite changes with both types of lenses.
Finally, we attempt to address the question of
which layers in the posterior wall of the globe are the
controllers of growth and which are the ones controlled. We cocultured normal scleral layers with either the other scleral layer or with choroid from eyes
in which the visual experience had been varied. We
found that the fibrous sclera appears to control the
rate of sulfate incorporation into cartilage GAGs, but
that the choroid also can influence both scleral layers.
MATERIALS AND METHODS
Animals and Visual Manipulations
White Leghorn chickens {Gallusgallus domeslicus), obtained as day-old chicks from Truslow Farms (Chestertown, MD), were reared in our animal facility on a
14:10-hour light:dark cycle. Care and use of the animals conformed to the ARVO Resolution on the Use
of Animals in Research.
Induction of Refractive Errors. To deprive eyes of
form vision, white translucent plastic diffusers were
glued to the feathers around one eye.18 To impose
refractive errors to provoke compensatory changes,
spectacle lenses of polymethylmethacrylate with a base
curve radius of 7 mm and an optic zone diameter of 10
mm in powers of ±15 D (Conforma Contact Lenses,
Norfolk, VA) were glued to rigid plastic washers
attached to Velcro rings, and complementary Velcro
rings were glued to feathers around the eyes, giving a
field of view of approximately 70° to 90°. Feed was
sifted to remove dust particles, and lenses were
cleaned approximately every 3 to 4 hours during the
light phase each day. Unless otherwise indicated, each
chick was fitted with a diffuser or lens over one eye;
the fellow eye was left uncovered.
Refractive Error Measurements
Refractive error was measured in chicks wearing spectacle lenses (chicks fitted with diffusers were assumed
to be highly myopic on the basis of many previous
studies). Chicks were anesthetized with a mixture of
1728
Investigative Ophthalmology & Visual Science, August 1997, Vol. 38, No. 9
chloral hydrate and sodium pentobarbital (0.3 g/ml
and 65 mg/ml, respectively). Mydriasis (and presumably cycloplegia) was obtained by administering 1
drop/min for 5 to 10 minutes of 10 mg/ml vecuronium bromide (Norcuron; Organon, West Orange,
NJ) and benzalkonium chloride (0.26 mg/ml) in saline. Refractive error was measured using a modified
Hartinger refractometer (ausjena, Jena, Germany);
data were not corrected for the artifact of retinoscopy.
Tissue Dissection and Explant Culture
The culture medium used in these experiments was
the chemically defined medium (N2) of Bottenstein. 19
This medium consists of Dulbecco's modified Eagle's
medium and Ham's F-12 with the addition of sodium
bicarbonate, sodium selenite, progesterone, putrescine, pyruvate, glutamine, and insulin. Antibiotics
used were penicillin, streptomycin, and amphotericin
B (Gibco, Grand Island, NY). Evidence that our culture methods maintained healthy tissue over the 48hour period used here is indicated by the finding that
isolated pieces of sclera cultured for 72 hours in continuously flowing N2 show consistently high levels of
sulfate incorporation into secreted GAGs throughout
the culture period. 20 In addition, pieces of sclera cultured for 48 hours have similar incorporation, regardless of whether the medium is refreshed at 24 hours
(compare results of experiments 3 and 4).
A punch 7 mm in diameter was taken from the
posterior sclera of each eye of chicks killed with an
overdose of intraperitoneal sodium pentobarbital.
The punch was located so that it nearly contacted the
central extremity of the pecten, such that the long
axis of the pecten formed a tangent to the circular
punch, which lay on the nasal side of the pecten. The
fibrous and cartilaginous scleral layers were separated
by peeling away the fibrous from cartilaginous sclera
in cold Ca ++ /Mg ++ -free Hank's balanced salt solution
(Sigma, St. Louis, MO) under a dissecting microscope.
In another set of birds, the choroid was isolated in
cold Ca ++ /Mg ++ -free Hank's balanced salt solution
by first peeling away the neural retina, then gently
brushing away the retinal pigment epithelium, and
finally peeling the choroid away from the sclera. Tissues were in the dissecting medium for approximately
5 to 30 minutes and then were transferred to tissue
culture medium (without insulin) until all dissections
were completed.
Scleral Growth Assays
Because we find greater variability in all label incorporation measures between individual animals than between the eyes of an individual, we organized the experiments so that the data collected are measures on
a treated eye compared with the untreated fellow eye
of the same individual. There may be a disadvantage to
this procedure, however. In treatment with spectacle
lenses, the refractive error of the fellow eye changes
slightly in the same direction as the lens-treated eye.21
If the same were true for incorporation of precursors
into GAGs, our procedure might understate the magnitude of the treatment effects.
We assessed the incorporation of Na 2 35 SO 4 into
GAGs as a reflection of the rate of proteoglycan synthesis.8 Scleral layers were incubated with Na235SO4
(10 //Ci/ml) for either 3 or 20 hours, digested overnight at 57°C in 0.5 ml of proteinase-K (protease type
XXVII, Sigma, in 10 raM EDTA, 0.1 M sodium phosphate [pH = 6.5]), centrifuged for 5 minutes at 7500g,
and divided into two aliquots. In one aliquot (200
//I), GAGs were precipitated for 1 hour at 37°C with
cetylpyridinium chloride in 2 mM Na 2 SO 4 in the presence of unlabeled chondroitin sulfate (1 mg/ml), captured by filtration (Whatman GF/F), and scintillation
counted using CytoScint (Fisher Biotech, Pittsburgh,
PA). In the other aliquot (50 fi\), DNA content was
measured by fluorimetry using Hoechst 33258 dye
(Polysciences, Warrington, PA).22
Precursor incorporation into protein and DNA
was measured by labeling scleral layers with 3 H-proline
(5 //Ci/ml) or 3 H-thymidine (5 //Ci/ml), respectively,23 for 20 hours. Subsequently, the tissues were
agitated in four changes of cold 5% trichloroacetic
acid, each lasting 24 hours, to remove unincorporated
radiochemical. In each experiment, we confirmed
that the last wash did not contain radioactivity above
background levels. Tissues were then digested in 0.5
ml of proteinase, centrifuged for 5 minutes at 7500g,
and divided into two aliquots. One aliquot (100 fi\)
was scintillation counted to assess precursor incorporation, and the other aliquot (50 /zl) was used to determine the DNA content by fluorimetry. Uptake of precursors was expressed as normalized to DNA content
to reflect the synthetic activity per cell. This was desirable because visual deprivation decreases the cell density in the posterior scleral region from which the
punches were taken.24 The range of counts for all
samples (measurements of sulfate, proline, and thymidine incorporation) was fibrous sclera, 550 to 5,230
cpm, cartilaginous sclera, 7,150 to 27,020 cpm, and
background 30 to 50 cpm.
We measured only the newly synthesized GAGs
that were incorporated into the scleral tissue; by separately assaying the culture medium in a few cases, we
estimate that the amount lost to the medium, which
we did not process, was small (roughly 10%) in cartilaginous sclera but larger in the fibrous sclera. Perhaps
because the proteoglycans from the fibrous sclera are
smaller, they diffuse out of the tissue more readily.
Data Analysis
As mentioned, uptake of 35S-sulfate, 3 H-proline, and
3
H-thymidine into tissues was normalized to DNA con-
1729
Vision Modulates Growth of Chick Scleral Layers
tent and values were expressed as femtomoles of radiochemical incorporated/ng DNA. We calculated femtomoles incorporated by comparing the sample of processed tissue with a standard well without tissue
containing a known quantity of radiochemical. For
comparisons between scleras of treated and untreated
eyes of the same bird, data are expressed as ratios of
the paired treated/control layers, after each datum
was divided by the amount of DNA in the sample. A
paired Student's Mest, comparing the ratios to a null
hypothesis of mean = 1, was performed for all data
expressed as ratios.
PROTOCOLS
Experiment 1: Form Deprivation and Recovery
From Form Deprivation
To assess the effect of form deprivation, day-old chicks
had one eye covered by a diffuser for 14 days (other
chicks had sulfate incorporation measured after 7 days
of form deprivation). To assess the effect of recovery
from form deprivation, chicks had one eye covered by
a diffuser for 11 days, followed by diffuser removal
and 3 days of normal vision. In all groups, the fellow
eyes were left untreated. In a similar study, the mean
refractive error after form deprivation exceeded —15
D; after 3 days of recovery, it was about —6 D.25 After
enucleation, we separated the fibrous and cartilaginous scleral layers of a posterior scleral punch. Precursor incorporation into GAGs, DNA, and protein of
the fibrous and cartilaginous layers was measured as
described above, after incubation with Na235SO4, 3Hthymidine, or 3H-proline for 20 hours. The number
of subjects in each group is indicated in Figure 4.
Experiment 2: Effect of Defocusing Spectacle
Lenses
Day-old chicks wore either —15 D lenses (n = 11),
which imposed hyperopic defocus, or +15 D lenses
(n = 13), which imposed myopic defocus (except at
very close distances); fellow eyes were left untreated.
On day 9, lenses were removed and refractive error
was measured. This was followed by enucleation and
separation of fibrous and cartilaginous scleral layers
of a posterior scleral punch. Paired fibrous and cartilaginous layers were then separately cultured in N2
for 20 hours with Na235SO4 and processed to quantify
incorporation into GAGs and DNA content.
Experiment 3: Tissue Interactions in Coculture
To assess whether the effects of different visual manipulations on the scleral layers were the result of the
independent actions on each layer of substances diffusing from elsewhere or whether one layer could influence the synthetic activities of other layers, we co-
Conditioning Tissues
Take punch,
dissect out:
-fibrous sclera
-cartilaginous sclera
-choroid
0 o
Measured Tissues
oo
Coculture tissues
for 20 hr
Discard
conditioning
tissues
Add label,
culture
for 20 hr
Digest conditioned
tissues and measure
sulfate incorporation
into GAGs
Normalize to DNA
content and express as:
expt(x) / control(c)
DNA
DNA (c)
FIGURE 2. Protocol for tissue coculture. Measured tissue consisted of fibrous and cartilaginous layers from normal eyes,
separated as described in methods. This yielded two pieces
of cartilaginous and two pieces of fibrous scleral tissue for
each animal. These pieces were cocultured for 20 hours with
different conditioning layers from the eyes of an animal that
had one experimental (form-deprived or recovering from
form deprivation) and one untreated fellow eye. Tissue combinations during coculture were conditioning fibrous with
measured cartilaginous sclera or vice versa, or either measured fibrous or cartilaginous sclera with conditioning choroid. After the 20-hour coculture, the conditioning tissues
were discarded and labeled sulfate was added to the existing
conditioned media for 20 hours (fibrous sclera) or for 3
hours (cartilaginous sclera).
cultured normal fibrous or cartilaginous tissue from
untreated eyes (measured tissues) with either the choroid or the complementary scleral layer (conditioning
tissues) from normal eyes, eyes wearing diffusers
(termed form-deprived eyes), or eyes that had previously worn diffusers (termed recovering eyes). The
visual manipulations for these animals were as described for experiment 1; the number of subjects in
each group is indicated in Figures 7 and 8.
As shown in Figure 2, the measured tissues consisted of fibrous and cartilaginous layers from both
normal eyes of 7-day-old chicks, separated as described
above. This yielded two pieces of cartilaginous and
two pieces of fibrous sclera for each animal. These
pieces were cocultured for 20 hours with different
layers (conditioning tissues) from both eyes of another animal (fibrous with cartilaginous or vice versa,
or either fibrous or cartilaginous with choroid). After
the 20-hour coculture, the conditioning tissues were
1730
Investigative Ophthalmology & Visual Science, August 1997, Vol. 38, No. 9
discarded and Na 2 35 SO 4 was added to the existing conditioned media for 20 hours (fibrous sclera) or 3
hours (cartilaginous sclera). To reduce the effect of
individual variability, we constructed these experiments so that the two eyes of one animal provided the
conditioning tissues compared (form-deprived versus
normal or recovering versus normal) and the two eyes
of another animal provided the normal tissues measured.
For one experiment, we tested conditioning tissues from recovering and untreated eyes on measured
tissues from recovering, rather than normal, eyes. To
retain the advantage just described of having the conditioning and measured tissues each come from a single animal, we obtained the conditioning tissue as follows. Diffusers with a small nasal opening that restricted vision to a small portion of the temporal retina
were placed over both eyes of 4-day-old chicks for 11
days (n = 7), resulting in myopic refractions on the
optic axis (mean refractive error: right eyes —13.8 D,
left eyes —15.8 D). Diffusers were then removed and
the eyes were permitted 3 days of normal vision. We
know that restoring vision to locally deprived parts of
the retina causes the refractions and the eye shape to
return to normal. 26
For another experiment, we again departed from
our paradigm by testing conditioning tissue from a
normal eye on measured tissue from a normal eye of
another animal; the measured tissue from the fellow
normal eye was incubated without any conditioning
tissue.
Experiment 4: Effect of Separation of Fibrous
From Cartilaginous Sclera
To assess the effect of separation of the scleral layers
per se, we used the protocol shown in Figure 3. Paired
normal eyes from chicks ages I ( n = 1 7 o r l 8 ) , 7 ( n
= 11), 8 (n = 18), 14 (n = 12), and 23 days old (n =
11) were enucleated and scleral punches taken. In
the punch from one eye of each animal (designated
initially separated), scleral layers were separated and
incubated separately in N2 for 20 hours. In the punch
from the other eye (designated initially intact), the
intact scleral punches were incubated in N2 for 20
hours, after which the scleral layers were separated.
Then, scleral layers were incubated separately in fresh
N2 with Na235SO4 for 3 or 20 hours (all ages), 3Hproline (n = 11; 7 days old), or 3H-thymidine (n =
10; 7 days old) for an additional 20 hours.
RESULTS
Experiment 1: Form Deprivation and Recovery
From Form Deprivation
Our principal discoveries are that sulfate incorporation into GAGs is affected in opposite directions in two
Cartilaginous
Initially
Digest
Tissues
Intact
Fibrous
Digest
Tissues
Initially
Separated
20 hr
Start
Radiopulse
40 hr
End
Radiopulse
FIGURE 3. Protocol for assessing the effect of separation of
scleral tissues on sulfate incorporation into GAGs. Tissues
were left initially intact (top) or were initially separated (bottom) during the first 20 hours in culture. Then, initially
intact tissues were separated, and all cartilaginous and fibrous scleral layers were measured with radioactive inorganic sulfate, thymidine, or proline for 20 hours. Tissues
were then processed to determine the amount of precursor
incorporation into GAGs, DNA, and protein, as well as the
DNA content. The arrowhead indicates that in certain experiments, cartilaginous sclera was labeled with radioactive
inorganic sulfate for only 3 hours.
respects. First, for a given visual manipulation (form
deprivation or recovery), the two scleral layers change
in opposite directions. Second, for a given scleral layer
(fibrous or cartilaginous sclera), the changes are in
opposite directions for form deprivation and recovery
from form deprivation. Specifically, in the cartilaginous layer from form-deprived eyes, the sulfate incorporation is nearly three times that of the untreated
fellow eye (291% at day 7 and 297% at day 14; Fig.
4A), whereas in the cartilaginous layer from recovering eyes, it is significantly decreased (to 81% of untreated eyes at 14 days of age; Fig. 4E). The opposite
is true of sulfate incorporation in the fibrous layer. In
form-deprived eyes it is decreased to 74% of untreated
eyes, and in recovering eyes it is increased to 177% of
untreated eyes.
This general bidirectional pattern is partly evident
in 3H-thymidine incorporation into DNA as well. In
scleral tissues from form-deprived eyes, 3H-thymidine
incorporation changes in the same direction and to
similar extents as does sulfate incorporation into
GAGs (to 262% of untreated in cartilage, down to
77% in fibrous; Fig. 4B). In scleral tissues from recovering eyes, the 3H-thymidine incorporation in cartilage changes in the opposite direction as during form
deprivation (down to 77%; Fig. 4F), but the recovering fibrous sclera does not show a significant change
(104% of normal).
The pattern of 3H-proline incorporation into protein in cartilaginous sclera also resembles that of sul-
Vision Modulates Growth of Chick Scleral Layers
1731
Form-depnvation
3-
A
a.
*
H Fibrous
B
T *
• Cartilaginous
-3
D
C
m
o
z
>
o
o
I
H
<t
Q.
OC
OC
T
-1.5
X
•D
(D
(S
3
£
sr
1-
-1
ss
•n
(D
-.75
.75*
*
0.5-
-0.5
n=41
n=11
n=7
n=23
z
a
IAL LAYEF
CD
How eye
o
*
1.5-
NSC
E
-2
2-
I eye
PRECURS
INCO
(feimtomoles radioche ica
;§
2
o
0
il eye
o
Experim
z
s>
>>
a
0)
-0.33
0.33GAG
DNA content
Protein
DNA
Recovery from form-deprivation
E
G
F
o>
J:
8
_o
1.5-
8.
>»
o 2
c
DC
xpe rim
o
2-
"5
o
HI
0.
o
E
&
111
*
T
1 -
.75-
*
—H«r
*
•
•
-2
-1.5
$
IWNWN
n=7
Ii?
*
0.5n=12
m
X
"D
n
rimer
OC
eye
Q
-3
H
*
n=6
-.75
In
-0.5
•<
CD
NTENTIN
POR/MION
ical/
^-
00 VN
3-
CO
c
m
>
)>
rr
3
(/)
n=25
-0.33
0.33GAG
DNA
Protein
DNA content
FIGURE 4. Responses of fibrous and cartilaginous scleral layers from eyes form-deprived from
day 1 to day 14 (A to D), from day 1 to day 7 {anmus, n = 18), and recovering from form
deprivation (E to H). DNA content represents data from sulfate-, thymidine-, and prolinelabeled tissues combined. Data shown are mean (±SEM). Vertical axis is logarithmic, so a
given percentage change is denoted by the same distance; this means that more molecules
of precursor are incorporated for a given distance above 1 than below 1. An asterisk indicates
statistical significance at the level of P < 0.05.
fate incorporation into GAGs. Incorporation increased to 145% in tissue from form-deprived eyes
and decreased to 81% in tissue from recovering eyes
relative to tissue from fellow untreated eyes (Figs. 4C,
4G). In contrast, the fibrous sclera from form-deprived
and recovering eyes incorporated significantly more
3
H-proline (179% and 213%, respectively). This suggests that both increased and decreased ocular elongation may involve increased protein synthesis in the
chick fibrous sclera.
An independent confirmation of the general pattern of opposite responses in the two scleral layers is
that the total amount of DNA per scleral disk shows
small nonsignificant trends that are opposite in cartilaginous and fibrous layers: form-deprived eyes, lower
in cartilage and higher in fibrous (Fig. 4D), and recovering eyes, higher in cartilage and lower in fibrous
(Fig. 4H). Only the decrease in cartilage from myopic
eyes is statistically significant. Thus, across groups, the
changes in DNA content tend to be opposite to the
changes in the rate of DNA synthesis, as evaluated by
thymidine incorporation. This pattern is probably a
consequence of the cartilage's enlarging by a greater
increase in extracellular matrix synthesis than in cell
division, so that the density of cells is reduced, whereas
in the fibrous layer the cell division increases more
than does the extracellular matrix synthesis.
Experiment 2: Effect of Defocusing Spectacle
Lenses
Eyes can be made to grow in the myopic direction not
only by form deprivation but also by allowing them to
compensate for hyperopic refractive error imposed by
negative spectacle lenses. In these experiments, we
find the same pattern of sulfate incorporation into
GAGs as in form-deprived eyes—that is, increased (to
150%) in the cartilaginous layer and decreased (to
68%) in the fibrous layer, relative to untreated fellow
eyes (Fig. 5A). We know that the lenses were being
compensated for because negative lens wear resulted
in a myopic mean refractive error of —5.1 D, but un-
Investigative Ophthalmology & Visual Science, August 1997, Vol. 38, No. 9
1732
changes in sulfate incorporation (Figs. 5B and 5D).
These results suggest that the same mechanisms are
used for growth in myopic and hyperopic directions,
whether caused by visual deprivation, by recovery from
its effects, or by lenses.
Negative lens wear
Experiment 3: Tissue Interactions in Coculture
GAG
DNA content
Positive lens wear
-0.33
GAG
DNA content
FIGURE 5. Sulfate incorporation into GAGs and DNA content
in fibrous and cartilaginous scleral layers in response to
negative (A,B) and positive (C,D) lens wear. Nine-day-old
chicks wore a lens over one eye since postnatal day 1. Data
shown are mean (±SEM). Vertical axis is logarithmic. An
asterisk indicates statistical significance at the level of P <
0.05.
treated fellow eyes had a mean refractive error of 1.2
D (Fig. 6).
Conversely, eyes can be made to grow in the hyperopic direction not only by being given normal vision after form deprivation (recovery) but also by
allowing them to compensate for myopic refractive
error imposed by positive spectacle lenses. Here, too,
we find the same pattern as in eyes recovering from
visual deprivation. Eyes wearing positive lenses had
sulfate incorporation in the cartilaginous layer decreased to 65% and sulfate incorporation in the fibrous layer increased to 133% relative to untreated
fellow eyes (Fig. 5C). Positive lens wear resulted in a
hyperopic mean refractive error of +11.2 D; untreated
fellow eyes had a mean refractive error of +1.0 D
(Fig. 6). As in the previous experiment, there were
nonsignificant trends in DNA content opposite to the
To ascertain whether the two scleral layers respond
oppositely because they have independent responses
to signals from another tissue or because one layer
determines the response of the other layer, we cocultured fibrous or cartilaginous sclera with the complementary scleral layer from form-deprived or recovering eyes (methods shown in Fig. 2). We found a
remarkable asymmetry between the effects of the scleral layers on each other (Figs. 7A and 7B).
The fibrous sclera effectively imposed the visual
conditions of its eye of origin on the cartilaginous
sclera with which it was cultured. This is shown by
three results. First, scleral cartilage from normal eyes
cocultured with fibrous sclera from form-deprived
eyes had 143% more sulfate incorporation into GAGs
than did cartilage cocultured with fibrous sclera from
untreated eyes (Fig. 7A, row B, right panel). Thus,
coculture with fibrous sclera (conditioning tissue)
from form-deprived eyes caused normal cartilage to
increase sulfate incorporation, making it similar to
cartilage from form-deprived eyes (compare Fig. 7A,
row B, right panel and Fig. 4A). Second, coculture
with fibrous sclera from eyes recovering from formdeprivation myopia caused sulfate incorporation to
decrease to 76% in the cartilaginous sclera from normal eyes, making it similar to cartilage from recovering eyes (compare Fig. 7A, row C, right panel and
Fig. 4E). These effects are bidirectional, as can be
seen by comparing the experimental tissues to those
cultured alone (Fig. 7A, left panel). If we express the
effect of fibrous sclera from deprived eyes on scleral
cartilage from normal eyes relative to being incubated
a>
o.
o
* •—|3SHH
•15
Q,
J~^
n=11
IT 1
n=13
a>
§
Q.
(0
S
+15
-8
-4
0
+
4
+8
+12
+16
Refractive Error (Diopters)
FIGURE 6.
Refractive errors of chicks wearing negative or positive lens over one eye {filled bars), and the untreated fellow
eyes {open bars). Nine-day-old chicks wore a lens over one
eye since postnatal day 1. Data shown are mean (±SEM).
An asterisk indicates statistical significance at the level of P
< 0.05.
1733
Vision Modulates Growth of Chick Scleral Layers
EFFECT OF FIBROUS SCLERA ON CARTILAGINOUS SCLERA
Conditioning
Tissues
Measured
Tissues
NORMAL
NORMAL
NONE
NORMAL
FORM-DEPRIVED
UNTREATED
NORMAL
NORMAL
1
FORM-DEPRIVED
n=12 1
RECOVERING
UNTREATED
* 1—^^H
RECOVERING
UNTREATED
NORMAL
NONE
n=12
n=6
RECOVERING
RECOVERING
n=7
20
0.75
1.0
1.50
1.25
Ratio of sulfate
incorporation into GAG
Sulfate incorporation into GAG
(femtomoles sulfate / ng DNA)
EFFECT OF CARTILAGINOUS SCLERA ON FIBROUS SCLERA
Conditioning
Tissues
Measured
Tissues
NORMAL
NORMAL
NONE
NORMAL
FORM-DEPRIVED
NORMAL
UNTREATED
NORMAL
RECOVERING
NORMAL
UNTREATED
NORMAL
RECOVERING
RECOVERING
UNTREATED
RECOVERING
A
B
C
D
n=12
i—
n=12
^W///////,
*
0.1
0.2
0.3
Sulfate incorporation into GAG
(femtomoles sulfate / ng DNA)
0.75
1.0
NORMAL
NONE
FORM-DEPRIVED
UNTREATED
n=6
n=7
I—
0
B
* rH
i—
1.25
RECOVERING
UNTREATED
1.50
Ratio of sulfate
incorporation into GAG
FIGURE 7. Sulfate incorporation into GAGs in (A) cartilaginous and (B) fibrous scleral layers
from paired normal eyes of the same animal cocLiltured with complementary scleral layers
(fibrous in the case of cartilaginous, cartilaginous in the case of fibrous) from eyes in
different visual conditions. Each bar represents the mean (±SEM) sulfate incorporation
into GAGs/ng DNA (left panels) and the mean ratio of the pairs (±SEM) (right panels) of
the measured tissues cocultured with the conditioning tissues shown at left. The bars in row
A are unfilled to emphasize that they represent a different ratio (cocultured with normal
conditioning tissue relative to no conditioning tissue). An asterisk indicates statistical significance at the level of P < 0.05. In both scleral layers, sulfate incorporation into GAGs (left
panels) in normal tissues cocultured with untreated tissues (rows B and C) is not significantly
different (two-sample Hest) from normal tissues cocultured with normal tissues (row A).
with no other tissue (that is, multiplying the "FormDeprived/Untreated" ratio by the "Normal/None"
ratio in Fig. 7A), the ratio is 1.60 (Form-Deprived/
Normal X Normal/None = 1.43 X 1.12 = 1.60). If
we form the equivalent ratio for the effect of the fibrous sclera from eyes recovering from deprivation,
the ratio is 0.85 (Recovering/Normal X Normal/
None = 0.76 X 1.12 = 0.85). Third, a similar decrease
was seen if the fibrous sclera from recovering and
untreated eyes was cocultured with scleral cartilage
from recovering eyes. This decrease occurs because
the cartilage from recovering eyes cocultured with untreated fibrous increased its incorporation to normal
levels (Fig. 7A, row D, left panel).
In contrast, the cartilaginous sclera did not im-
pose the visual conditions of its eye of origin on the
fibrous sclera. Instead, cartilage from normal eyes inhibited fibrous sclera sulfate incorporation into GAGs
relative to being cultured alone, and cartilage from
either form-deprived or recovering eyes reduced it
42% more (Fig. 7B, right panel). Taken together,
these results are consistent with the fibrous sclera's
being the determinant of the pattern of response of
both layers of the sclera.
However, the effect of coculture with choroid of
different origins suggests that the choroid also affects
the cartilage. As was the case with coculture with fibrous sclera, sulfate incorporation into GAGs of cartilaginous sclera from normal eyes was increased (to
117%) by coculture with choroid from form-deprived
Investigative Ophthalmology 8c Visual Science, August 1997, Vol. 38, No. 9
1734
EFFECT OF CHOROID ON CARTILAGINOUS SCLERA
Conditioning
Measured
Tissues
Tissues
NORMAL
NORMAL
NONE
NONE
FORM-DEPRIVED
NORMAL
UNTREATED
NORMAL
RECOVERING
UNTREATED
10
15
20
0.50
25
0.75
1.0
1.25
Sulfate incorporation into GAG
Ratio of sulfate
(femtomoles sulfate)
incorporation into GAG
1.50
EFFECT OF CHOROID ON FIBROUS SCLERA
Conditioning
Measured
Tissues
Tissues
NORMAL
NORMAL
NONE
NORMAL
FORM-DEPRIVED
NORMAL
UNTREATED
NORMAL
RECOVERING
NORMAL
UNTREATED
NORMAL
C
0.1
0.2
0.3
0.4
0.5
0
1
n=12
-I
* w//////m
;, ,
1
B
I
B
I
A
n=7
n=12
u
0.50
1.0
1.50
Sulfate incorporation into GAG
Ratio of sulfate
(femtomoles sulfate)
incorporation into GAG
NORMAL
NONE
FORM-DEPRIVED
UNTREATED
2.0
FIGURE 8.
Sulfate incorporation into GAGs in (A) cartilaginous and (B) fibrous scleral layers
cocultured with choroid from eyes in different visual conditions. Data shown are mean
(±SEM) sulfate incorporation into GAGs/ng DNA {left panels) and mean ratio of the pairs
(±SEM) {right panels), as in Figure 7. The bars in row A are unfilled to emphasize that they
represent a different ratio (cocultured with normal choroid relative to no conditioning
tissue). An asterisk indicates statistical significance at the level of P < 0.05. In both scleral
layers, sulfate incorporation into GAGs {left panels) in normal tissues cocultured with untreated tissues (rows B and C) is not significandy different (two-sample Mest) from normal
tissues cocultured with normal tissues (row A).
eyes (Fig. 8A, row B, right panel) and decreased (to
59%) by coculture with choroid from recovering eyes
(Fig. 8A, row C, right panel). However, because choroid from normal eyes inhibited cartilage from normal
eyes to 78% of the sulfate incorporation level of cartilage incubated alone (Fig. 8A, row A, right panel), we
view the choroid results as being composed only of
inhibition, as can be seen by comparing the experimental tissues to those cultured alone (Fig. 8A, right
panel). Thus, the choroid from form-deprived eyes
inhibits the cartilage to 91% relative to being incubated alone (Form-Deprived/Normal X Normal/
None = 1.17 X 0.78 = 0.91; Fig. 8A, rows A and B,
right panel), and the choroid from recovering eyes
inhibits it to 46% (Recovering/Normal X Normal/
None = 0.59 X 0.78 = 0.46; Fig. 8A, rows A and C,
right panel). In contrast, the response of cartilage to
coculture with fibrous sclera is truly bidirectional.
The effect of choroid on fibrous sclera is also unidirectional: the choroid from normal eyes stimulates
sulfate incorporation in fibrous sclera from normal
eyes to 133% of fibrous incubated alone (Fig. 8B, row
A, right panel), and the myopic choroid has the same
effect (Fig. 8B, row B, right panel), whereas the choroid from recovering eyes stimulates it to 264% (Fig.
8B, row C, right panel). The fact that the choroid
inhibits the cartilage and stimulates the fibrous is further evidence for the opposite responses of the two
scleral layers and for the independence of their responses to extrinsic chemical signals.
Experiment 4: Effect of Separation of Fibrous
From Cartilaginous Sclera
The sulfate incorporation into GAGs in the fibrous and
cartilaginous scleral layers from normal eyes changed in
opposite directions with age. Sulfate incorporation in
the fibrous sclera decreased 10-fold after postnatal day
1 and showed smaller, if any, changes thereafter (Fig.
9A). In contrast, sulfate incorporation in the cartilaginous sclera increased monotonically from day 7 to day
23 (Fig. 9B). These age-dependent changes in sulfate
incorporation into GAGs are statistically significant by
Vision Modulates Growth of Chick Scleral Layers
•
Layers initially intact
H Layers initially separated
1735
midine incorporation increased to 148% compared to
initially intact layers of fellow normal eyes (significant
by two-sample Kest [P < 0.05]).
DISCUSSION
7-8
-'
14
23
0
Age (days)
9. Precursor incorporation into GAGs, DNA, and
protein in (A) fibrous and (B) cartilaginous scleral tissues
either initially separated or initially left intact during first 20
hours in culture. Scleral tissues were obtained from paired
untreated eyes of chicks ages 1 (n = 17 or 18), 7 (n = 11),
8 (re = 18), 14 (n = 12), and 23 (re = 11) days. Data shown
are mean (±SEM). A two-sample t-test was performed for
unpaired comparisons. An asterisk indicates statistical significance (two-sample Hest) at the level of P< 0.05. The "x"
indicates a single outlier not included in statistical analysis.
FIGURE
linear regression for fibrous (slope < 0, P < 0.0001)
and cartilaginous tissues (slope > 0, P < 0.0001),
whether they were separated initially or separated after
20 hours in culture together (initially intact).
Mechanical separation of the sclera from normal
eyes into its two component layers by itself caused an
increase in sulfate incorporation into GAGs, as assessed by comparing tissue separated initially with that
separated after 20 hours in culture together. (To measure the sulfate incorporation into GAGs, the scleral
layers were separately incubated with label.) In general, both layers of initially separated sclera had
greater sulfate incorporation than those that had been
initially intact (for example, in 7- and 8-day-old chicks,
sulfate incorporation in cartilage increased to 146%
and in fibrous to 142%, both of which were significant
by a two-sample Hest [P < 0.05]).
Precursor incorporation into DNA and protein
showed a similar, statistically significant, pattern in the
cartilaginous but not in the fibrous layer. Initially separated cartilaginous layers from normal eyes had 3 Hproline incorporation increased to 153% and 3H-thy-
These results show that the two layers of the chick
sclera respond differently in all situations examined
and that both layers must be studied to understand
how vision controls the growth of the chick eye. Specifically, we have shown two main effects.
First, when the rate of elongation of the eye is
altered by visual manipulations, the cartilaginous and
fibrous layers of the eye show opposite modulations
of growth, as indicated by sulfate incorporation into
GAGs. In the cartilaginous layer of the sclera, the precursor incorporation into GAGs increases with more
rapid ocular elongation (visual deprivation or wearing
negative lenses) and decreases with less rapid ocular
elongation (recovery from visual deprivation or wearing positive lenses). In the fibrous layer of the sclera,
the opposite changes occur. Thus, the changes in the
fibrous sclera are in the same direction as in formdeprived and recovering tree shrews.1'1"16 Our results
are also consistent with those of Gottlieb et al2<1 showing that visual deprivation causes the cartilaginous
sclera to thicken and the fibrous sclera to thin and
with evidence of decreased scleral thickness in formdeprived tree shrews27'28 and monkeys,29 and in myopic humans. 30
Second, the effects of visual conditions on growth
of the scleral cartilage appear to be controlled by the
fibrous sclera. The stimulatory effect of form deprivation and the inhibitory effect of recovery from form
deprivation can be mimicked by coculturing normal
cartilaginous sclera with fibrous sclera from form-deprived or recovering eyes. Furthermore, the inhibitory
effect on the scleral cartilage of recovery from form
deprivation can also be mimicked by coculture with
choroid from recovering eyes. In contrast, the fibrous
sclera is stimulated by coculture with choroid, especially from recovering eyes, but is inhibited by donor
cartilaginous sclera regardless of the visual conditions
of the eye of origin.
Does Precursor Incorporation Into GAGs in
Separated Scleral Layers Reflect Normal
Growth Processes?
We have shown consistent and opposite changes in
incorporation of sulfate into GAGs in the two scleral
layers. We view these as probably indicating corresponding changes in proteoglycan synthesis. We recognize that in some proteoglycans, the level of sulfation may change independently of core protein synthesis. In cartilage, however, the predominant
proteoglycan, aggrecan, is fully sulfated.31 Further-
1736
Investigative Ophthalmology 8c Visual Science, August 1997, Vol. 38, No. 9
more, because the GAGs in sclera (predominantly the
cartilaginous sclera) have a half-life of 10 days,32
whereas our measurements of sulfate incorporation
into GAGs took no longer than 20 hours, the rate of
incorporation reflects mostly new net synthesis, at least
in the case of cartilaginous sclera.
One of the difficulties in interpreting this work is
that the experiments necessitated physical separation
of the scleral layers to assess their separate synthetic
activities. We must consider, therefore, how precise
the separation was and how great an effect this separation is likely to have had on the sclera. To answer the
first question, the separation is unlikely to have left
significant amounts of cartilage on the fibrous layer,
because the variability of precursor incorporation into
GAGs in cartilage and fibrous sclera is comparable,
although the cartilage has 100-fold greater radioactivity (Fig. 9). If the separation left fibrous sclera on the
cartilaginous sclera, this would have had a negligible
effect on our measurements of the cartilage for the
same reason. Indeed, faulty separation cannot be responsible for the changes in precursor incorporation
into GAGs in our experimental eyes, because the
amount of DNA hardly changes and the changes that
occur are in the opposite direction.
Furthermore, it appears that the act of separation
does not drastically affect the response of the tissues,
because the precursor incorporation into DNA, protein, and GAGs is similar in tissues separated at the
start of incubation compared to ones incubated together first and separated later (Fig. 9). This similarity
is maintained over a 4-week range of ages, even though
the absolute levels change 10-fold in the fibrous sclera
over that period and the degree of adhesion between
the scleral layers changes enormously (layers come
apart easily at hatching but are difficult to separate at
3 weeks of age).
Nonetheless, there is an effect of separation of
the layers. Those separated initially consistently incorporate somewhat more sulfate than those separated
later (Fig. 9). We cannot distinguish whether this is
caused by synthetic activities related to repair of tissue
damage or by the fact that both layers are released
from a mutual inhibition. If we deliberately increased
the tissue damage by making a second 4-mm-diameter
punch within the 7-mm punch used in the experiments reported here, the rate of sulfate incorporation
into the unseparated sclera nearly doubles.
However, we are encouraged to think that our
opposite results from fibrous and cartilaginous sclera
are real phenomena because they are so precisely
mimicked by coculturing the separate layers from normal eyes with their complementary layers from eyes
with different visual experiences and are consistent
with results from coculture with choroids on scleral
punches that had not had their layers separated 33 (see
Opposite Responses in Cartilaginous and Fibrous
Sclera).
Relation to Compensation for Spectacle Lenses
We find that negative and positive lenses modulate
precursor incorporation into scleral GAGs in opposite
directions, both in the cartilaginous and fibrous sclera.
This confirms a recent study on unseparated sclera.17
Because the +15 D lenses would cause profound myopic defocus for all but the closest objects and the —15
D lenses would cause hyperopic defocus that would
not be fully compensated by accommodation, we take
our findings as evidence for bidirectional growth modulation of the sclera rather than as a variant of deprivation myopia, as some have attempted to do. 34 ' 35 We
discuss this issue more fully elsewhere. 3637
Furthermore, the similarity of responses to lenses
and to form deprivation and recovery suggests that
these manipulations, despite the differences they produce in visual processing, share a final path in the
control of ocular elongation.
Opposite Responses in Cartilaginous and
Fibrous Sclera
Three simple humoral explanations could account for
the opposite responses of the two scleral layers to deprivation or defocus. First, two different sets of extrinsic
signals from the retina or elsewhere could separately
guide the growth of the two layers. Second, a single
set of signals could have opposite effects on the two
layers. Third, one layer could receive the extrinsic signals and control the other layer. Our results suggest
that this last alternative may suffice. We find that coculture of normal scleral cartilage with fibrous sclera
from form-deprived or recovering eyes causes growth
effects similar to those seen in the cartilage of eyes
that were themselves form-deprived or recovering, respectively. On this basis, one could argue that cartilage
need only respond to the fibrous sclera and that the
fibrous sclera could be the only scleral layer that responds to extrinsic signals.
However, we find that the choroid from formdeprived and recovering eyes influences cartilaginous
sclera in the same direction as does the fibrous sclera.
We argue, however, that they have different roles, the
fibrous sclera's having both stimulatory and inhibitory
roles and the choroid's having an inhibitory role. This
latter result is consistent with a study of the effect of
choroid-conditioned medium on sulfate incorporation into GAGs of pieces of sclera, which showed a
dose-dependent inhibition by choroids from recovering eyes and no stimulation from choroids from
visually deprived eyes.33* Finally, the fact that the co* In this experiment, because the scleral layers were not separated, the precursor incorporation into GAGs measured would
largely reflect the cartilaginous sclera, although the fibrous sclera
could have been a modulatory influence.
Vision Modulates Growth of Chick Scleral Layers
culture with normal fibrous sclera causes an increase
in the sulfate incorporation into GAGs of the scleral
cartilage, but coculture with normal choroid causes a
decrease, argues that these two tissues play distinct
roles. These findings do not identify either the choroid or the fibrous sclera as the source of the signaling
molecules. Either tissue might be acting as a sponge,
accumulating the secretions of yet another tissue.
In summary, these coculture studies make plausible the notion that the retina or retinal pigment epithelium produces diffusable signals that act on the
choroid and fibrous sclera, and that these tissues in
turn control the growth of the cartilaginous sclera.
Beyond this humoral explanation, there might be
a mechanical explanation for the opposite responses
of the cartilaginous and fibrous scleral layers. It is well
known that rhythmic mechanical forces result in a
increase in proteoglycan synthesis of cartilage (review
by Urban 38 ). Such forces cause hyperpolarization in
chondrocytes but depolarization in fibroblasts.39 Perhaps changes in intraocular pressure, 20 ' 40 either diurnal or caused by fluctuations in ocular accommodation, provoke opposite changes in proteoglycan synthesis in chondrocytes and fibroblasts.
Finally, there is evidence that the cartilaginous
and fibrous layers of the sclera should be viewed as a
single tissue, with cells at the boundary between the
layers switching identity between chondrocytes or fibroblasts as circumstances demand—that is, increased chondrogenesis during increased ocular elongation and increased fibrogenesis during decreased
ocular elongation. At the boundary with the fibrous
sclera, scleral cartilage from form-deprived eyes incorporates unusually large amounts of sulfate41 and the
boundary between the layers becomes indistinct,42 suggesting active chondrogenesis. In bovine fibrocartilaginous tendon, compressive loading increased the
synthesis of large proteoglycans such as aggrecan, the
cartilage proteoglycan, and decreased the synthesis of
small proteoglycans, such as decorin, found in the
fibrous sclera; unloaded tendon showed the opposite
changes. 43
Why does the fibrous sclera decrease its rate of
precursor incorporation into both DNA and GAGs
when the eye is elongating especially rapidly? In other
connective tissues, decreased GAG synthesis is associated with decreased mechanical stiffness.44 This may
be the case in the fibrous sclera as well, at least in the
tree shrew, as shown by the association of decreased
GAG synthesis with increased extensibility during applied constant force in form-deprived eyes.14'15'45 Furthermore, if the sclera is artificially weakened by treatment with lathyritic agents, tree shrew ocular elongation is enhanced. 46 Thus, it seems plausible that the
stiffness of the fibrous sclera must be reduced for the
eye to elongate rapidly and that this is done by de-
1737
creased GAG synthesis, presumably accompanied by
increased proteolysis. Cartilage, in contrast, increases
its GAG synthesis during rapid ocular elongation (the
turnover also increases somewhat compared to normal32) . In the chick, the loss of stiffness of the fibrous
sclera of form-deprived eyes might be compensated
for by increased stiffness of the cartilage, associated
with the increased GAG synthesis. This might help
explain why lathyritic agents do not enhance ocular
elongation resulting from form deprivation in
chicks.46 These remodeling activities in both layers
may be mediated by changes in the activation and
inhibition of gelatinases, as has been shown for both
chicks and tree shrews during form deprivation.47'48
Differences Between Birds and Mammals
The pattern of results presented here resolves the apparent difference between birds and mammals shown
by other studies. The fibrous sclera of the chick shows
decreased sulfate incorporation into GAGs during the
increased ocular elongation brought about by form
deprivation, as has been shown for the fibrous sclera
of the tree shrew.15 The difference in the chick is that
it also has a layer of cartilage, which shows opposite
responses. When the sulfate incorporation into GAGs
is measured without separating the scleral layers, the
cartilage greatly dominates because its proteoglycans
are much more highly sulfated, giving the impression
that the chick sclera shows opposite responses to those
of the mammalian sclera.
In fact, the anthropomorphic tendency to regard
the avian sclera as aberrant and distinct from the mammalian sclera ought to be resisted. Walls49 argues that
a sclera with cartilage, being stiffer than a fibrous
sclera and thus permitting larger and less spherical
eyes, is the general vertebrate form and that scleral
cartilage has been lost only in three groups (snakes,
salamanders, and nonmonotreme mammals). He suggests that in mammals with exceptionally large eyes
(e.g., whales and elephants), the lack of cartilage requires the sclera to be so thick that it occupies most
of the volume of the eye. Finally, even the presence
of cartilage in the avian sclera is not so great a difference from the mammalian sclera as it appears, in that
mammalian sclera also expresses two of the three principal phenotypic markers for cartilage.50
If we combine Walls' speculations with the ones
we made earlier in this discussion, we can view the
fibrous sclera as needing to soften to grow. As a consequence, in mammals rapid elongation leaves the sclera
vulnerable to losing the rigidity necessary to withstand
expansion driven by the normal intraocular pressure;
if this happens, a posterior staphyloma may result. In
contrast, the general vertebrate pattern of a sclera
reinforced with cartilage may be able to grow without
this risk. This might explain why chicks with 20 to 30
1738
Investigative Ophthalmology & Visual Science, August 1997, Vol. 38, No. 9
D of myopia for long periods have stable eye shapes 51
and why chicks in which the sclera is weakened by
lathyritic agents do not develop longer eyes after visual
deprivation. 46 Furthermore, one could view the Russian therapy of injecting foreign substances behind
the eye to stimulate inflammatory connective tissue
growth as a step toward giving humans the structural
benefits of a cartilaginous sclera.
In conclusion, how the retina adjusts the growth
of the sclera, and thereby the size and shape of the
eye, remains a mystery, but all evidence points to a
single solution for both birds and mammals, one that
involves bidirectional modulation of scleral remodeling to enhance or reduce the rate of ocular elongation
during growth, thereby continuously adjusting the eye
toward functional emmetropia.
Key Words
cartilage, choroid, hyperopia, myopia, proteoglycans
Acknowledgments
The authors thank Debora L. Nickla and Sek-Jin Chew for
a critical reading of an earlier version of this manuscript
and Tadayo Kusakari for the use of Figure 1.
References
1. Goss DA, Criswell MH. Myopia development in experimental animals: A literature review. AmJOptom Physiol
Opt. 1981; 10:859-869.
2. Raviola E, Wiesel TN. Animal model of myopia. NEngl
JMed. 1985;312:1609-1615.
3. Wallman J. Retinal control of eye growth and refraction. Prog Retinal Res. 1993; 12:133-153. .
4. Laties AM, Stone RA. Some visual and neurochemical
correlates of refractive development. Visual Neurosci.
1991;7:125-128.
5. Criswell MH, Gross DA. Myopia development in nonhuman primates—A literature review. Am J Optom
Physiol Opt. 1983;60:69-81.
6. Holden AL, Hodos W, Hayes BP, Fitzke FW. Myopia:
induced, normal and clinical. Eye. 1988;2:S242-S 256.
7. Goss DA, Wickham MG. Retinal-image mediated ocular growth as a mechanism for juvenile-onset myopia
and for emmetropization—A literature-review. Doc
Ophthalmol. 1995; 90:341-375.
8. Rada JA, Thoft RA, Hassell JR. Increased aggrecan
(cartilage proteoglycan) production in the sclera of
myopic chicks. Dev Biol. 1991; 147:303-312.
9. Christensen AM, Wallman J. Evidence that increased
scleral growth underlies visual deprivation myopia in
chicks. Invest Ophthalmol Vis Sci. 1991;32:2143-2150.
10. Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and
relation to emmetropization. Vision Res. 1987;
27:1139-1163.
11. SivakJG, Barrie DL, Callender MG, Doughty MJ, Seltner RL, WestJA. Optical causes of experimental myopia. In: Bock G, Widdows K, eds. Myopia and the Control
ofEye Groiuth (Ciba Foundation Symposium 155). Chiches-
ter: John Wiley & Sons; 1990:160-177.
12. Nickla DL, Gottlieb MD, Christensen AM, et al. In
vitro proteoglycan synthesis is higher in sclera from
myopic eyes and lower in sclera from recovering eyes.
ARVO Abstracts. Invest Ophthalmol Vis Sci. 1992;
33:1054.
13. Christensen A, Wallman J. Modulation of regional
scleral DNA and protein synthesis underlies deprivation myopia and recovery. Proc 9th Int Cong Eye Res.
Helsinki, 1990.
14. Norton XT, Rada JA. Reduced extracellular matrix in
mammalian sclera with induced myopia. Vision Res.
1995;35:1271-1281.
15. Reeder AP, McBrien NA. Investigation of scleral metabolism during the development of experimental myopia in the tree shrew. ARVO Abstracts. Invest Ophthalmol Vis Sci. 1994;35:1801.
16. McBrien NA, Lawlor AP. Increased proteoglycan synthesis in the sclera of tree shrew eyes recovering from
form deprivation myopia. ARVO Abstracts. Invest Ophthalmol Vis Sci. 1995;36:S760.
17. Nickla D, Wildsoet C, Wallman J. Compensation for
spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res.
1997; 16:320-326 (plus erratum 16:624-625).
18. Wallman J, Ledoux C, Friedman MB. Simple devices
for restricting the visual fields of birds. Behav Res Meth
Instrument. 1978; 10:401-403.
19. Bottenstein JE. Defined media for dissociated neural
cultures. Curr Meth Cell Neurobiol. 1983; 4:107-130.
20. Nickla DL. Diurnal Rhythms and Eye Groiuth in Chiclis.
New York: City University of New York; 1996. PhD
dissertation.
21. Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks.
Vision Res. 1995;35:1175-1194.
22. Lambarca C, Paigen K. A simple, rapid, and sensitive
DNA assay procedure. Anal Biochem. 1980; 102:344352.
23. Celis JE, ed. Cell Biology: A Laboratory Handbook. New
York: Academic Press; 1994.
24. Gotdieb MD, Joshi HB, Nickla DL. Scleral changes in
chicks with form deprivation myopia. Curr Eye Res.
1990;9:1157-1165.
25. Wallman J, Wildsoet C, Xu A, et al. Moving the retina:
choroidal modulation of refractive state. Vision Res.
1995; 35:37-50.
26. Xu A. Local Choroidal and Scleral Mechanisms of Recovery
From Partial Myopia. New York: City University of New
York; 1992. Master's thesis.
27. Kang R, Norton T. Alteration of scleral morphology
in tree shrews with induced myopia. ARVO Abstracts.
Invest Ophthalmol Vis Sci. 1993;34:1209.
28. Norton T, Kang R. Morphology of tree shrew sclera
and choroid during normal development, induced
myopia, and recovery. ARVO Abstracts. Invest Ophthalmol Vis Sci. 1996;37:324.
29. Funata M, Tokoro T. Scleral changes in experimentally myopic monkeys. Graefe's Arch Clin Exp Ophthal-
mol. 1990;228:174-179.
Vision Modulates Growth of Chick Scleral Layers
30. Curtin BJ, Teng CC. Scleral changes in pathological myopia. Trans Am Acad Ophthalmol Otolaryngol. 1957; 62:777790.
31. Kuetter KE, KimuraJH. Proteoglycans: an overview. /
Cell Biochem. 1985; 27:327-336.
32. Rada JA, Achen V, Perry CA. Scleral extracellular matrix
43.
changes in experimental myopia. ExpEyeRes. 1996;63:S9.
33. Gottlieb M, Nickla D, Wallman J. Evidence for a modulatory role of the choroid on the sclera in developing eyes.
ARVO Abstracts. Invest Ophthalmol Vis Sci. 1993; 34:1209.
44.
34. Zadnik K, Mutti DO. How applicable are animal myopia models to human juvenile-onset myopia? Vision
Res. 1995; 35:1283-1288.
35. Mutti DO, Zadnik K, Adams AJ. Myopia: the nature
versus nurture debate goes on. Invest Ophthalmol Vis
Sci. 1996; 37:952-957.
45.
36. Wallman J. How is emmetropization controlled? Results of research on experimental myopia. In: Tokoro
T, ed. Sixth International Conference on Myopia. Hakone,
46.
Japan: Springer-Verlag; 1996; in press.
37. Wallman J. How many myopias? In: Christen Y, Doly
M, Droy-Lefaix MT, eds. Vision et Adaptation (vol. 6 of
47.
Les Seminaires Ophthalmologiques d'IPSEN). Paris: Elsevier; 1995:140-150.
38. Urban JP. The chondrocyte: a cell under pressure. Br
48.
fRheumatol. 1994; 33:901-908.
39. Wright MO, Stockwell RA, Nuki G. Response of
plasma membrane to applied hydrostatic pressure in
chondrocytes and fibroblasts. Connective Tissue Re49.
search. 1992; 28:49-70.
40. Nickla DL, Wallman J. The diurnal rhythms of intraoc50.
ular pressure and ocular elongation are altered in
myopic chick eyes. ARVO Abstracts. Invest Ophthalmol
Vis Sci. 1995; 36:413.
41. Rada JA, Matthews AL, Brenza H. Regional proteoglycan synthesis in the sclera of experimentally myopic
51.
chicks. ExpEyeRes. 1994;59:747-760.
42. Kusakari T, Sato T, Kaneko M, Funata M, Wake K,
1739
Tokoro T. Possible interaction between the fibrous
sclera and cartilaginous sclera in chicks with visual
deprivation myopia. In: Chew SJ, Weintraub J,
eds. Fifth International Conference on Myopia. Toronto: Myopia International Research Foundation;
1994.
Koob TJ, Clark PE, Hernandez DJ, Thurmond FA,
Vogel KG. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 1992; 298:303-312.
Tammi M, Paukkonen K, Kiviranta I,JurvelinJ, Saamanen A-M, Helminen HJ. Joint loading-induced alterations in articular cartilage. In: Helminen HJ, Kiviranta I, Tammi M, Saamanen A-M, Paukkonen K, Jurvelin J, eds. foint Loading. Bristol: Butterworth;
1987:64-68.
Phillips J, McBrien N. Form deprivation myopia: elastic properties of the sclera. Ophthalmol Physiol Opt.
1995; 15:357-362.
McBrien NA, Norton TT. Prevention of collagen crosslinking increases form-deprivation myopia in tree
shrew. ExpEyeRes. 1994;59:475-86.
Rada J, Brenza H. Increased latent gelatinase activity
in the sclera of visually deprived chicks. Invest Ophthalmol Vis Sci. 1995; 36:1555-1565.
Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;
37:1380-1395.
Walls GL. The Vertebrate Eye and its Adaptive Radiations.
Bloomfield Hills: Cranbrook Institute of Science; 1942.
Poole AR, Pidoux I, Reiner A, Coster L, Hassell JR.
Mammalian eyes and associated tissues contain molecules that are immunologically related to cartilage
proteoglycan and link protein. J Cell Biol. 1982;
93:910-920.
Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek
LA. Local retinal regions control local eye growth and
myopia. Science. 1987;237:73-77.