experiment 3 synthesis of an alum kal(so4)
advertisement

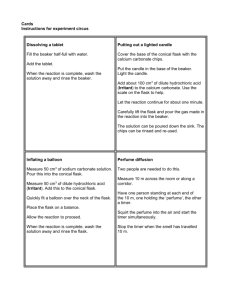
EXPERIMENT 3 SYNTHESIS OF AN ALUM KAL(SO4)212H2O INTRODUCTION Potassium aluminum sulfate dodecahydrate, KAl(SO4)212H2O, belongs to a class of inorganic compounds called alums (which is accented on the first syllable). The composition of alum is represented by the general formula M+M3+(SO4)212H2O. In this formula, M+ is a monoatomic ion such as Na+, K+, Tl+, or Ag+; or is NH4+. Although the name alum sounds as if the compound must contain aluminum, the M3+ might be from any of several main-group metals (p block elements) and transition metals (d block elements) that form triply charged ions: Al, Ti, V, Cr, Mn, Fe, Co, Ga, In, and Ir. Some examples are NaAl(SO4)212H2O, which is a component of some baking powders; NH4Al(SO4)212H2O, which is used in dyeing textiles; and KCr(SO4)212H2O, which is used in tanning leather. The term double salt also describes KAl(SO4)212H2O. A double salt is an ionic compound that contains two different cations or two different anions. In aqueous solution, double salts give positive tests for all three of the ions they contain. KAl(SO4)212H2O would give positive tests for K+, Al3+, and SO42. Hydrate is another term that describes KAl(SO4)212H2O. A hydrate is a substance that contains a specific number of water molecules per formula unit in its solid form. Hydration is common with ionic compounds, for example Na2SO410H2O and CoCl26H2O. The water of hydration is included when calculating the compound’s formula mass. The water molecules are part of the crystal of a compound along with cations and anions, but they can break out of the crystal when it is heated. This dehydration might occur at a specific temperature characteristic of the particular compound. If the compound is heated in a relatively closed container, the escaping water might dissolve the remaining salt forming a liquid solution. Consequently, this combination of dehydration and dissolving looks like melting; the temperature range at which it occurs (the solid-to-liquid transition) can be measured in the same way that a melting point is measured. Reactions Involved in the Synthesis Metals dissolve in water or aqueous solutions only by reacting with water or some other component of the solution. The reaction converts the metal to a positive ion. Aluminum dissolves in acids by reacting with H3O+ and dissolves in bases by reacting with OH. The reaction you will use to dissolve aluminum involves OH from KOH: 2 Al(s) + 2 OH- (aq) +6 H2O 2 Al(OH) 4 (aq) + 3 H2(g) (3-1) Next, you will add sulfuric acid to the solution containing Al(OH) 4. As H3O+ reacts with Al(OH)4, insoluble Al(OH)3 forms and precipitates. As more acid is added, the precipitate Al(OH)3 dissolves: Experiment 3 3-1 Al(OH) 4 (aq) + H3O+(aq) Al(OH)3(s) + 2 H2O(l) (3-2) Al(OH)3(s) + 3 H3O+(aq) Al3+(aq) + 6 H2O(l) (3-3) The product forms and precipitates when the solution containing Al 3+, K+ (from KOH), and SO42 (from H2SO4) is cooled: K+(aq) + Al3+(aq) + 2 SO 24 (aq) + 12 H2O (l) KAl(SO4)212 H2O(s) (3-4) EQUIPMENT NEEDED balance beakers Büchner funnel hot plate clamp conical funnel Erlenmeyer flasks filter flask filter paper—12.5cm and 7.0cm graduated cylinder melting point capillary tubes ring stand rubber O-ring slit stopper temperature probe spatula watch glass glass stirring rod CHEMICALS NEEDED isopropyl alcohol Al foil ice 6 M H2SO4; sulfuric acid 3M KOH; potassium hydroxide thymol blue solution PROCEDURE Dissolving Al by Reaction with KOH 1. Weigh a piece of aluminum foil on the laboratory balance. Remove some Al from it or add more Al to it until you have ~0.5–0.6 grams; record the mass. Tear the Al into pieces about the size of large postage stamps and put these pieces into an Erlenmeyer flask. 2. Take a beaker to the hood, measure out ~20mL of 3M KOH solution into the graduated cylinder provided, and pour the measured solution into the beaker. Take the beaker back to you bench area and pour the 3M KOH solution into the flask containing the aluminum foil. Within a few minutes the Al and KOH will begin to react producing hydrogen; this reaction will become progressively more vigorous. Do not handle the flask; the reaction is so exothermic that the entire flask, including the neck, will become extremely hot. After the obvious evolution of hydrogen ceases, let the flask stand for a few minutes more to ensure complete reaction of the Al. Then cool the flask slightly by wiping the neck with a wet sponge. Cool the flask further under cold, running water until you can handle the entire flask comfortably. Experiment 3 3-2 3. If the solution in the flask is absolutely clear, proceed to the next step in the synthesis. If the solution is not absolutely clear, filter it by gravity filtration. Collect the filtrate in another Erlenmeyer flask. Discard the filter paper in the regular trash container. Converting Al(OH)4 To Al3+ by Reaction with H2SO4 4. To the clear solution in an Erlenmeyer flask, add 16.0 mL of 6M H2SO4 a few milliliters at a time. After each addition, swirl the flask to mix the contents. Initially, Al(OH) 3 will precipitate; then some of it will dissolve as more H2SO4 is added. If solid sticks to the inside wall of the flask, wash it down with a few drops of H2SO4. After you have added all the H2SO4, gently heat the flask for several minutes; have the liquid barely bubbling. If the amount of H2SO4 added to the reaction mixture was sufficient for the amounts of Al and KOH you used, all of the solid in the flask should dissolve. 5. If solid remains, continue the gentle heating for several more minutes; do not increase the intensity of the heating. If solid still remains, gradually add more H2SO4 dropwise with gentle heat until all of the solid dissolves and you have a clear solution; this should not require more than one mL (about 20 drops) of H2SO4. Precipitating KAl(SO4)212H2O by Cooling 6. Place the flask containing the reaction mixture into a beaker of ice water; be sure the water in the beaker is at least as deep as the solution in the flask, and that there is plenty of ice in the beaker. Occasionally stir the solution with a glass rod. After crystals of the product begin to form, cool the mixture for at least another ten minutes; continue to stir it occasionally. While you wait for the product to precipitate, set up the equipment for filtration. Collecting, Washing, and Drying KAl(SO4)212H2O. 7. Set up a Büchner funnel apparatus. Clamp the filter flask to a ring stand and connect the sidearm of the flask to the vacuum line with a long piece of pressure tubing. Place the funnel on top of the flask, and insert a piece of filter paper. Open the vacuum line so that it is pulling air through the funnel. Wet the filter paper in the funnel with some water from a wash bottle until it forms a seal with the base of the funnel. Swirl the flask with your product so that the solid is dispersed in the solution, then pour it into the funnel. Wash any remaining solid left in the flask into the funnel with a small amount of icecold water. The water must be as cold as possible; since the product is slightly soluble in water, using cold water ensures that the amount of product that is dissolved away is minimized. When no more drops of water are seen coming out of the tip of the funnel, turn off the vacuum. 8. The washing of the precipitate is done to purify the product by removing the excess ions present in the starting materials from the product. This step takes advantage of the differing solubility properties of the impurities and the product; the ionic impurities are all readily soluble in water, while the alum is only slightly soluble in water. One of the ionic impurities is hydronium ion; we can monitor the progress of the washing by using an indicator to detect the presence of hydronium ion. Put 2 or 3 drops of distilled water into a small test tube, add one drop of thymol blue solution, then remove the funnel and tubing from the filter flask and transfer a few drops of the filtrate (the liquid in the filter flask) into the thymol blue solution with a disposable pipet. The first filtrate that you collect should Experiment 3 3-3 give a pink color with the indicator showing that an excess of acid was used in the reaction. After testing this first filtrate, keep the test tube of indicator and filtrate to use to make color comparisons with subsequent washings. 9. Pour the filtrate out of the filter flask into a beaker (you will be pouring this into a waste container at the end of the lab period), rinse the flask with water, reconnect the tubing and replace the funnel with your product. Open the vacuum line and wash the product with 10 mL of ice-cold water. Test the filtrate as above with a fresh water-and-thymol blue solution in a clean test tube. If the thymol blue solution turns pink, repeat this washing step. If the thymol blue turns yellow or perhaps a pale orange, the product has been adequately washed. When all the excess acid has been washed out of the product, you can be confident that any other excess starting material has also been washed away. When you complete the filtering and washing, the filtrate can be discarded by washing it down the drain. The thymol blue solutions can also be discarded by washing them down the drain. 10. Obtain 20 mL of isopropyl alcohol in a dry container. With the vacuum line open, pour about 5 mL of isopropyl alcohol over the entire surface of the solid in the funnel. Repeat this procedure three more times. The isopropyl alcohol washes away the water; isopropyl alcohol is much more volatile than water and it will evaporate fairly rapidly to give a dry product. Allow the vacuum to pull air through your product for a few minutes to speed up the drying process. 11. Obtain a large piece of filter paper. Lift the wet filter paper and product from the funnel and use a spatula to scrape the solid onto the larger piece of dry filter paper. Use a spatula to spread the solid out as much as possible; cut through it repeatedly with a spatula to expose new solid surface and hasten drying. If the large piece of filter paper becomes quite wet, transfer the solid to another large piece of filter paper and continue cutting through and spreading the solid for a minute. 12. Transfer a small amount of the solid to a watch glass. Spread it out completely so that it can dry thoroughly in a few minutes. While it dries, set up the equipment for measuring the melting point. Disposing of Acid Waste. When your TA instructs you to do so, take the beaker containing the acidic filtrate from the initial vacuum filtration (step 9) to the hood and pour it in the Acid Waste container. Experiment 3 3-4 Determining the KAl(SO4)212H2O. Note: “Solid to Liquid” Transition Temperature of Wait until you are sure that the small sample of the product is completely dry before performing this part. 13. Use an ice bath to calibrate the temperature probe, following the procedure from EXPERIMENT 1. 14. Place a small beaker on wire gauze on a ring on a ring stand with a hot plate beneath the beaker. Obtain a capillary tube. 15. Push the powder into a mound. Press the open end of the capillary tube into the mound of powder once. Turn the tube upright and repeatedly tap the sealed end against the desktop until all the powder falls to the bottom of the tube. Again, press the open end of the tube into the heap of powder once and tap the bottom of the tube against the desktop until all the powder falls to the bottom of the tube. Repeat this process until there is solid in the bottom of the tube to a height of about 5 mm. Use a rubber o-ring to hold the capillary tube onto the temperature probe so that the bottom of the capillary tube is even with the end of the temperature probe. Figure 3-1. Determining the melting point. 16. Set up an apparatus like FIGURE 3-1, using a hot plate instead of the burner. Note: the beaker may be placed directly onto the hot plate, so the iron ring and wire gauze are not needed. Put the temperature probe into a slit stopper and clamp it onto the ring stand. Put clear tap water into the beaker to a great enough depth that the tip of the temperature probe is completely immersed in the water. Be sure that the open end of the capillary tube is above the water level. Also, have the rubber o-ring (rubber band in the figure) above the water level. 17. Begin to heat the bath and stir it gently and continuously. Record the temperature at which you first see solid change to liquid; the solid will seem to collapse as it begins to melt and will be translucent or transparent, rather than opaque. Record the temperature at which all solid finally changes to liquid. If the melting range is more than 3C, you were probably heating the water bath too rapidly. Repeat the measurement with a fresh sample of product in a fresh capillary tube. The narrowness of this range and the accuracy of your result depend on the purity, including dryness, of your product. While you are carrying out this step, you should occasionally cut through and redistribute your remaining product to facilitate drying. Dispose of the used capillary tube in the wastebasket. Experiment 3 3-5 Determining Yield. 18. Place a piece of weighing paper on a balance. Zero the balance, and then place the alum product on the weighing paper. Record the mass of the alum. Disposal of Product. After the mass of the alum has been determined, it may be thrown into a wastebasket. Experiment 3 3-6 EXPERIMENT 3 REPORT SHEET Name: _______________________________________ Date:__________ Mass of Al foil used Moles of Al used Volume of 3M KOH used Moles of KOH used Mass of KOH used Volume of 6M H2SO4 used Moles of H2SO4 used Mass of H2SO4 used Limiting reactant Theoretical yield of KAl(SO4)212H2O, moles Theoretical yield of KAl(SO4)212H2O, grams Actual yield of KAl(SO4)212H2O, grams % yield of KAl(SO4)212H2O “Solid to Liquid” transition temperature range Experiment 3 3-7 Laboratory Notes Exp. 3 Synthesis of an Alum Chemistry 111 You may work in pairs on this experiment. Changes in Procedure and Helpful Hints Step 2: You may get the reaction started by heating the flask on a hot plate. As soon as the reaction starts to bubble vigorously, turn off the hot plate! Step 3: The filtration works better if you wet the filter paper thoroughly with water before pouring in the reaction mixture. Step 6: This step takes a while; you can use the time to start working on your theoretical yield calculations. 10/10 Experiment 3 3-8