biochemistry10
advertisement

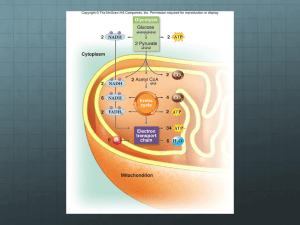
Subject: biochemistry Lec# 10 Dr. Karadsheih Date: 3-3-2011 Continuing with oxidative phosphorylation from last lecture: Last time we talked about electron flow which is utilized to pump H+ from the matrix to the intermembrane space. As a result, proton motor force was created resulting from the electrical and chemical tension that was created at both sides of the inner membrane. Protons have no other way to cross the inner membrane from the intermembrane space back to the matrix than ATP-Synthase complex, producing ATP in the process. ATP-Synthase Complex is formed from multi-subunits (node, rotator, ….) In order for the rotator to rotate once complete cycle, it will need 12 protons to pass through it, inducing conformational changes and releasing 3 ATP molecules in the process. So we conclude, the proton motive force is behind ATP synthesis. We know that respiration is an oxidation reaction of the food. Oxidation is always accompanied by reduction. Reduction is removal of electrons that are accepted by co-factors. Co-factors like: NADH (mainly) or FADH2. Oxidation happens mainly in the mitochondria (Krebs cycle) and some of it in the cytoplasm (glycolysis) NADH is produced in the cytoplasm. P.S. the inner membrane of the mitochondria is impermeable to NADH, so there should be special mechanism to transport NADH from cytoplasm to inside the mitochondria. Glycolysis in the cytoplasm produces NADH carrying electrons which should be transported to the electron transport chain inside the mitochondria. Each glycolysis produces 2 NADH Each NADH produces 3 ATP Q. How does the NADH that was produced in the cytoplasm reach inside the mitochondria?! A. We have two shuttle mechanisms: 1- The Glycerol-3-phospahte shuttle: (Operated mainly in the skeletal muscles and brain) Electrons from NADH are transported to dihydroxyacetone phosphate (a glycolitic intermediate) which is reduced to glycerol-3-phosphate with the help of an enzyme called glycerol-3-phosphate dehydrogenase (cytoplasmic enzyme) Now glycerol-3-phosphate enters the outer-membrane of the mitochondria towards the inner-membrane. On the inner membrane there is a membrane bound enzyme called glycerol-3phosphate dehydorenase (very similar to the previous one, but this one is a membrane bound enzyme). It re-oxidizes glycerol-3-phosphate back to dihydroxyacetone phosphate. Then electrons are transported to FAD reducing it to FADH2 and then electrons are transported to Coenzyme Q (part of the electron transport chain) Note: Because the electrons using the glycerophosphate shuttle enter the electron transport pathway at the level of Coenzyme Q , the electrons can only be used to synthesize a maximum of two ATP, instead of the maximum of three ATP derived from NADH formed inside the mitochondria. 2- Malate Aspartate Shuttle: (operated mainly in the liver and heart) Involves malate and aspartate to transport electrons across the mitochondrial inner membrane. Oxaloacetate in the cytoplasm is converted to malate by malate dehydrogenase, oxidizing NADH to NAD+.. The malate enters the mitochondria using an exchanger protein that must also transport α-ketoglutarate in the opposite direction (this protein is called antiporter). The malate is then oxidized to oxaloacetate by the mitochondrial malate dehydrogenase, resulting in formation of NADH, which can then enter the electron transport pathway. This shuttle conserves all of the energy in the NADH electrons, and allows the synthesis of three ATP (under optimum conditions) from NADH generated in the cytoplasm. Note: antiporters are special proteins that allow the entry of one molecule in exchange of another. Return of the oxaloacetate to the cytoplasm requires a separate transporter, which exchanges aspartate for glutamate. Oxaloacetate is transformed to become aspartate by an enzyme called aspartate amino-tansferase. P.S. we will talk about these reactions later in more details when we study metabolism The main difference between both shuttles is how many ATP each produces: The Glycerol-3-phospahte shuttle 2( FADH2) 2 ATP Malate Aspartate Shuttle 2( NADH) 3 ATP Poisons and Inhibitors of the Electron Transport Chain: 1- Rotenone: fish poison and insecticide ->inhibits electron transport between NADH and coQ ( complex I) 2- Antimycin A: antibiotic (inhibits complex III) 3- Carbon Monoxide (MO): inhibits cytochrome C oxidase (complex IV) 4- Cyanide: very lethal (complex IV) One way Cyanide poising could be treated is to oxidize some hemoglobin to give cyano-hemoglobin which prevents cyanide from reaching cytocrome C oxidase. 5- Oligomycin: blocks ATP-synthase, as a result protons can’t pass and ATP synthesis is inhibited. Protons concentration will increase dramatically in the intermembrane space and a steep gradient is established. Because protons continue to accumulate in the intermembrane space and their passage back to the matrix is blocked, oxidation phosphorylation is blocked as well, and the electron transport chain doesn’t function anymore. 6- Atractyloside: inhibits antiporter on the inner-membrane that permits ATP to exit the mitochondria. Uncoupling agents: Uncoupling is defined as a condition in which the rate of electron transport can no longer be regulated by an intact chemiosmotic gradient. The electron transport system is uninhibited due to complete and irreversible dissipation of the chemiosmotic gradient. it involves some kind of leakage of protons from intermembrane space back to the matrix without having to pass through ATP-synthase. It causes hyperthermia (increasing body temperature). 1- 2,3-dinitrophenol: when it enters the intermembrane space of the mitochondria, it gets protonated thus making it impossible to maintain a proton gradient. The uncoupling of electron transport from ATP synthesis allows rapid oxidation of Krebs substrates, promoting the mobilization of carbohydrates and fats, since regulatory pathways are programmed to maintain concentrations of those substrates at set levels. Since the energy is lost as heat, biosynthesis is not promoted, and weight loss is dramatic. This made it possible for this substance to be used as a drug for losing weight, but studies have shown that it might cause blindness to some patients, so it was withdrawn from the market. 2- Uncoupling Proteins: found in very small quantities on the inner-membrane of the mitochondria. Those proteins are present in high concentration in newborn babies in brown fat tissues (around the neck) for the purpose of producing heat Brown fat (thermogenic) is also found in hibernating animals to help them stay warm during hibernating season. Most of the proteins of the mitochondria are created from nuclear DNA. In Oxidative Phosphorylation there are almost 120 polypeptides, most created from nuclear DNA and 13 polypeptides from Mitochondrial DNA. Those 13 polypeptides are divided as follows: 7 in complex I, 1 in complex III, 3 in complex IV, 2 in ATP-synthase complex). Note: we all inherit our mitochondria from our mothers because mitochondria of sperm is excluded out. Diseases & Disorders Associated with Mitochondria: Most diseases that are related to the mitochondria are also related to oxidative phosphorylation and are called mitochondrial myopathy. Mitochondrial myopathy is caused by defects in the 13 polypeptides created from mitochondrial DNA. -Apoptosis: Cell’s programmed Auto-destruction. One of the causes of apoptosis is leakage of the outer-membrane of the mitochondria. Cytochrome C from the inner-membrane migrates to the cytoplasm and activates proteolytic enzymes that degrade most proteins of the cell which initiate programmed cell death. Best of luck, Osama Afaneh