Copper Cycle Lab: Reactions & Observations
advertisement
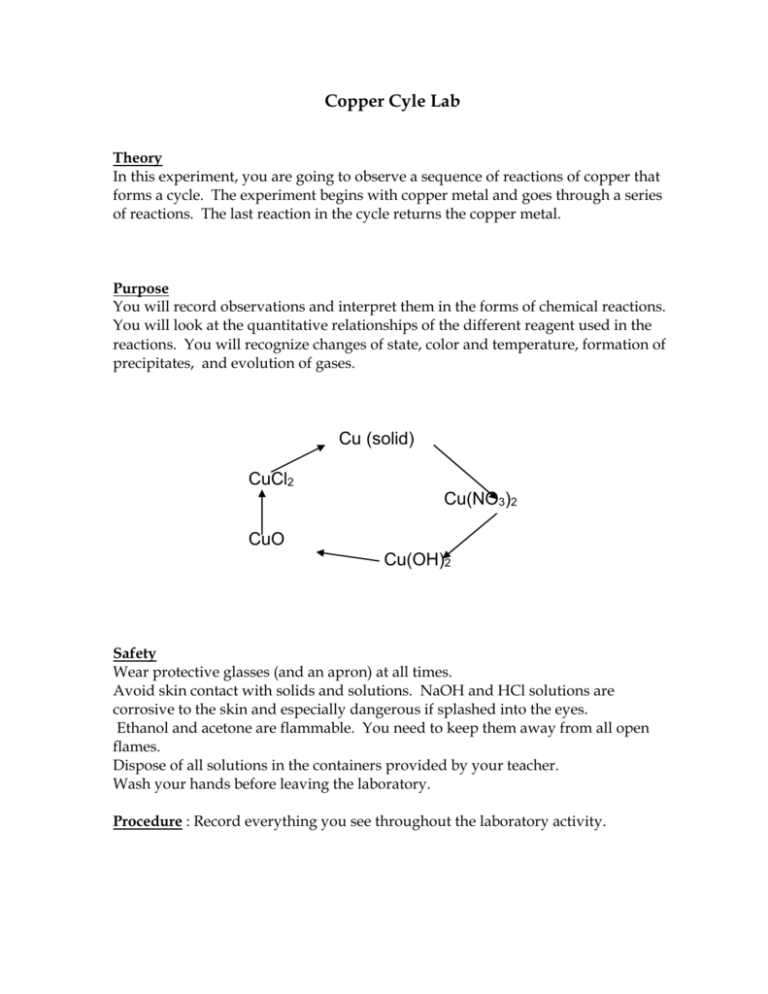
Copper Cyle Lab Theory In this experiment, you are going to observe a sequence of reactions of copper that forms a cycle. The experiment begins with copper metal and goes through a series of reactions. The last reaction in the cycle returns the copper metal. Purpose You will record observations and interpret them in the forms of chemical reactions. You will look at the quantitative relationships of the different reagent used in the reactions. You will recognize changes of state, color and temperature, formation of precipitates, and evolution of gases. Cu (solid) CuCl2 Cu(NO3)2 CuO Cu(OH)2 Safety Wear protective glasses (and an apron) at all times. Avoid skin contact with solids and solutions. NaOH and HCl solutions are corrosive to the skin and especially dangerous if splashed into the eyes. Ethanol and acetone are flammable. You need to keep them away from all open flames. Dispose of all solutions in the containers provided by your teacher. Wash your hands before leaving the laboratory. Procedure : Record everything you see throughout the laboratory activity. 1. (Teacher demo). In the fume hood, dissolve a small piece of copper wire (about .5g) in 4.0 ml of concentrated nitric acid (16M HNO3) in a 250 ml beaker. After the Cu is completely dissolved, add about 100ml of distilled water. Or use some Cu made from last year. (One student will feel the side of the beaker and report to the group.) The reaction for this step is: 4 HNO3 + Cu ==> Cu(NO3)2 + 2 NO2 + 2 H2O 2. A 0.1M Cu(NO3)2 stock solution was prepared earlier. You need to obtain 2-ml of this solution and put it into a large test tube. Add to this 0.6-ml (12 drops) of 2.0M NaOH slowly. Then stir gently with a glass stirring rod. (Caution: avoid contact with the sodium hydroxide as it burns the skin). Record your observations including cautiously feeling the test tube. The reaction for this step is: Cu(NO3)2 + 2 NaOH ==> Cu(OH)2 + 2 NaNO3 3. Clamp an iron ring to the ring stand and place a wire-gauze on it. Set a 100-ml beaker 1/2 full of distilled water. Heat with a bunsen burner but do not let it boil. Place the reaction test tube in the water bath. Stir gently while heating. When the transformation looks complete, remove the burner (turn it off), put the test tube in the rack and continue stirring for 1 minute and then allow any solids to settle. (When you have finished the minute of stirring, you may cool the test tube by placing it is another 100 mL beaker containing cool water.) The reaction for this step is: Cu(OH)2 + heat ==> CuO + H2O 4. Centrifuge the product (as per instructions). Then, using a pipette, draw off the top liquid (supernatant) and dispose of it in the sink: DO NOT LOSE ANY OF THE SOLID WHILE PIPETTING. Add 15 drops of the hot distilled water to wash the solid (precipitate). Tap the tube to mix and then allow the solid to settle once again. Again, centrifuge and remove the supernatant but not the solid. 5. Now to this precipitate, add 8 drops of 6M HCl while stirring. (Caution: avoid contact of both skin and clothing with the HCl). Tap the test tube to mix. The reaction for this step is: CuO + 2 HCl ==> CuCl2 + H2O 5. Now take your test tube to the fume hood and add one Al nail. Add 2 drops of water and 2 drops of 6M HCl. The final reaction is: CuCl2 + Al --> AlCl3 + Cu 2 6. When no hydrogen evolution can be detected, decant the supernatant liquid and transfer the copper to container indicated by washing it out of the test tube with a water bottle. 7. Clean your station and all your glassware and wash your hands before leaving. **Pre-lab: Go to WebAssign and complete assignment Data|Observations This is a qualitative lab so there are no number measurements. You should make a table to record all the observations for this lab. You might consider "the compound (added and formed)", "its formula" and "its color" along with other observations. Maybe use two columns with #1 being what you added or did to the current compound and #2 being the observations that happened. Calculations Because this is qualitative, there are no calculations per say. Redraw the cycle above (take a whole page) but add colors to each product formed and next to the arrows add what reagent was added to cause the reaction. Questions 1. Suppose the initial weight of the copper sample was 0.54 grams, and the recovered weight of copper was 0.50g. Find the % yield of copper in this series of reactions. 2. Why is this series of reactions often call the "copper cycle"? 3. What observations did you make that indicated a chemical reaction had taken place? Go through each step to answer this question. 3 References (This lab is adapted from the following sources) http://chemmovies.unl.edu/chemistry/labs/LABS01c.html http://chemmovies.unl.edu/chemistry/smallscale/SS016.html http://www.pc.maricopa.edu/chemistry/151LLWeb/A%20Cycle%20of%20Coppe r%20Reactions%20lab.htm 4









