Scientific Notation & Density Worksheet - Chemistry
advertisement

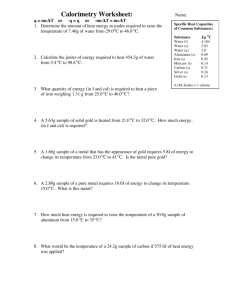
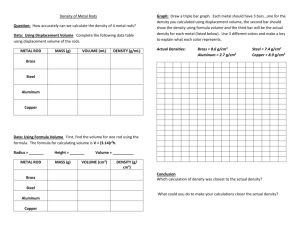
Name: ____________________________________________ Period: _______ Chemistry X Scientific Notation and Density Problems Part 1. Write the following numbers using scientific notation. 1) 53200 __________________ 6) 0.00032 __________________ 2) 980700 __________________ 7) 203 __________________ 3) 0.187 __________________ 8) 0.0485 __________________ 4) 390 __________________ 9) 0.000005 __________________ 5) 0.00298 __________________ 10) 2000000 __________________ Part 2. Write the following numbers using normal notation. 11) 1.2 x 109 _______________________________ 12) 3.45 x 10-3 _______________________________ 13) 5.6 x 102 _______________________________ 14) 8.21 x 105 _______________________________ 15) 9.7 x 10-5 _______________________________ Part 3: Solve the density problems written below. You must show all of your work to receive full credit for each problem. 16. A piece of tin has a mass of 16.52 g and a volume of 2.26 mL. What is the density of the tin? 17. A man has a 50.0 mL bottle completely filled with 163 g of a slimy green liquid. What is the density of the liquid? 18. A sealed 2500 mL flask is full to capacity with 0.36 g of a substance. Determine the density of the substance. 19. What is the mass of an object that has a density of 8 g/mL and a volume of 64 mL? 20. Different kinds of wood have different densities. The density of pine is generally about 0.5 g/mL. What is the mass of a 800 mL piece of pine? 21. What is the volume of 325 g of metal with a density of 9.0 g/mL? 22. Diamonds have a density of 3.5 g/mL. What is the volume of a diamond that has a mass of 0.10 g? 23. If a liquid has a volume of 620 mL and a mass of 480 g, what is its density? 24. A sample of a substance with a mass of 85 g occupies a volume of 110 mL. What is the density of the substance? 25. The density of a piece of brass is 8.4 g/mL. If its mass is 510 g, find the volume of brass. 26. The density of copper is 8.9 g/mL. You have a piece of copper with a volume of 23.4 mL. What is the mass of the piece of copper? 27. Mercury has a density of 13.6 g/mL. If you have 225 g of mercury, what is the volume of the mercury? 28. If 75.0 g of a liquid has a volume of 62.4 mL, what is the liquid’s density? 29. A cube of iron has a mass of 15.9 g. If iron has a density of 7.86 g/mL, what is the volume of the iron cube? 30. The element osmium is one of the densest substances on Earth. The density of osmium is 22.57 g/mL. What is the volume of a sample of osmium that has a mass of 4700 g? 31. Lead has a density of 11.3 g/mL. A piece of lead has a volume of 32.5 mL. What is the mass of the piece of lead? 32. The density of pure silver is 10.5 g/mL. If you have a 5.25 g of silver pellets, what is the volume of the silver? 33. What mass of water (in grams) will fill a tank 100 cm long, 50 cm wide, and 30 cm high? Remember, the density of water is 1.0 g/cm3. 34. A rock has a mass of 56.9 g. The rock is placed in a graduated cylinder containing 60 mL of water. The volume of the rock and the water is 78.2 mL. What is the density of the rock? 35. A solid metal rod is placed in a graduated cylinder containing 35.2 mL of water. The volume of the water and metal rod is 42.8 mL. If the rod has a mass of 20.5 g, what is the density of the metal rod?