Grade Level:

(Effect of pH on Umbionium shells)
Grade Level:
Junior high school or freshman biology classes
Subject:
Biology
Prepared By:
Jill Wojtanowicz and Heather Bartos
Overview and Purpose:
Ocean acidification is a rising issue in the field in environmental sciences. In this experiment we will
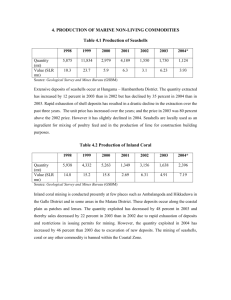
Illinois Learning Standards Addressed: determine the effects that acidity has on aquatic organisms through observing the physical changes that occur when umbonium seashells are placed in varying pH environments. There will be five
13.B.3f Apply classroom-developed criteria to determine the effects of policies on local science and technology issues experimental groups and one control group. In each group, 5 seashells will undergo experimentation.
The five experimental groups include placing these seashells in the following: 1 cup of dawn soap, 1
(e.g., energy consumption, landfills, water quality).
12.E.3b Describe interactions between solid earth, oceans, cup of 2% milk, 1 cup tomato juice, 1 cup coca-cola, and 1 cup lemon juice. In the control group, five seashells will be placed in 1 cup of deionized water. The seashell’s weight, texture, and color will be atmosphere and organisms that have resulted in ongoing changes of Earth (e.g., erosion, El Nino). recorded every other day for 2 weeks in order to determine the effects that pH has on seashells.
Objectives:
The students will:
11.A.3a Formulate hypotheses that can be tested by collecting data.
11.A.4b Conduct controlled experiments or simulations to test hypotheses
-Learn how to use a pH meter
-Understand the effects of acidity on aquatic organisms
-See how varying pH levels effects a species of umbonium seashells
Background Information:
Ocean acidification is the process of decreasing pH of the Earth’s oceans due to uptake of anthropogenic
11.A.4c Collect, organize and analyze data accurately and precisely.
carbon dioxide in the atmosphere. Dissolving carbon dioxide (CO2) into seawater increases the concentration of hydrogen ions (H+); thus adding acidity to the ocean. This acidity can affect the aquatic life- especially oceanic calcifying organisms. Acidity leads to the dissolution of calcium ions. Ultimately, this may effect the entire food chain.
Set-Up Instructions: Materials Included in Kit: deionized magnifying glass, pH meter, scale water, tomato juice, Coca Cola, lemon juice. Six umbonium seashells will also be placed on the lab table.
Activity Timeline:
Day 1: Students will assign each of the six seashells to their independent variable; in this case, the solution in which they will submerge it. Using the pH meter, the students will
Additional Notes:
(Effect of pH on Umbionium shells)
record the pH’s of the six solutions. Next, they will measure each of the seashells weight using a scale. Using the magnifying glass, they will then observe the seashell’s color, over pattern, and number of lines. Both quantitative and qualitative data will be recorded on chart in a lab handout. The students will then place the seashells into their corresponding solution (1-6). All of these beakers will then be placed in a refrigerator for a week.
Day 7: Approximately seven days later, they will take out each of their seashells and record the seashell’s weight, color, overall pattern, and number of lines. Difference should be noted.
This experiment can continue an additional week to see even more extreme results.
Safety Concerns: Additional Materials Needed: post seven days; milk may be contaminated at this point.
Additional Notes: