Fizzy Drink Stoichiometry Lab Name: Period: Materials: 1 – Dixie
advertisement

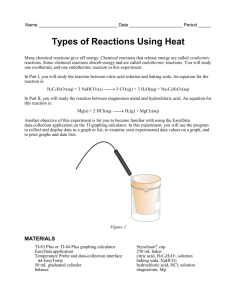
Fizzy Drink Stoichiometry Lab Materials: 1 – Dixie Cup Lemonade Mix Name: 1 – Balance Baking Soda Period: 1 – Mixing Spoon 1 – Weigh boat Citric Acid 3 – Ingredient spoons 2 – Larger Cups Instructions: 1) Make sure all of your materials are dry, if not; dry them with a paper towel before you begin. 2) Mix 2 spoonfuls of lemonade mix with a cup full of water. 3) Fill the Dixie cup with ¼ of Lemonade. Taste it. Record your observation and taste. 4) Mass out 0.5 g of citric acid and put it into the Dixie cup with ¼ of Lemonade. Taste it. Record your observation and taste. Pour the remaining Lemonade out. 5) Mass out 0.5 g of baking soda and put it into the Dixie cup with ¼ of Lemonade. Taste it. Record your observation and taste. Pour the remaining Lemonade out. Trial Ingredients Observations Taste 1 ¼ Dixie cupful of Lemonade 2 ¼ Dixie cupful of Lemonade + 0.5 g Citric Acid (C6H8O7) 3 ¼ Dixie cupful of Lemonade + 0.5 g Baking Soda (Sodium Bicarbonate, NaHCO3) 6) If you combine citric acid with Sodium Bicarbonate, they will react in solution to create sodium citrate (Na C6H7O7), water, and carbon dioxide. Write the chemical equation for this reaction. __________ + _________ -> ________ + ______ + _______ Citric Acid Sodium Sodium Citrate Water Carbon Dioxide Bicarbonate Part II If you have exactly 1.0 grams of citric acid (C6H8O7), how many grams of baking soda (NaHCO3) should be added to make fizzy Lemonade drink that is “just right”. 7) Convert 1.0 grams of citric acid (C6H8O7) (MM = 192 g/mol) into moles. 8) Using mole conversions go from moles of citric acid (C6H8O7) to moles of baking soda (NaHCO3). 9) Convert the moles of baking soda (NaHCO3) (MM = 88 g/mol) to grams. 10) Fill a Dixie cup with Lemonade. Pour the Lemonade into the large empty cup (to prevent bubble-over of mixture). 11) Mass out the grams of baking soda you just calculated, 1.0 g grams of citric acid, and mix them together with a Dixie cupful of Lemonade in the larger cup. 12) Mix and taste your concoction. Record your observation in the data table. Trial Ingredients Observations Taste 4 1 Dixie cupful of Lemonade + 1.0 g of citric acid + _____ g baking soda 13) How did you drink turn out? 14) How could you modify your recipe to make it better? 15) How can stoichiometry help us create a fizzy drink? 16) Where in your life have you done something similar to this experiment? 17) Where does stoichiometry show up in chemistry? 18) If you have 10.0 g of citric acid with enough baking soda, how many moles of carbon dioxide will you produce? a. Calculate moles of citric acid: b. How many moles of carbon dioxide will be produced: 19) If you have 10.0 grams of baking soda with enough citric acid, how many mole of carbon dioxide will you produce? 20) Looking at 19 and 20, which one produced less CO2? Why? 21) If you mixed 10 grams of each reactant in a container, would both be completely used up? Why or why not? 22) Assume the mole ratio of citric acid to baking soda was 1:3, it should take 1 gram of citric acid for 3 grams of baking soda for a complete reaction. Is this correct? Why or why not