Experiment #13: Archimedes' Principle
advertisement

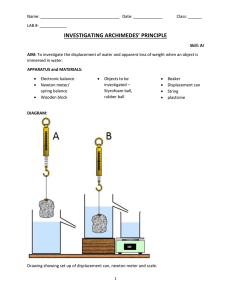
Experiment 13: Archimedes’ Principle Purpose (1) To observe Archimedes’ Principle aka the buoyant force. (2) To measure densities of a liquid and solid. Apparatus Laboratory Balance, Dial Calipers; a box of samples and a wooden rod; beakers; a graduated cylinder vessel; a supply of liquids; cleaning supplies. Theory (A) Archimedes’ Principle “An object immersed in liquid, fully or partially, experiences an upward buoyant force. This force is equal to the weight of the volume of liquid displaced by the object.” B Vdispl ρliq g = upward buoyant force = volume of liquid displaced by the object. = density of the liquid. = the acceleration of gravity ARCHIMEDES’ PRINCIPLE (1) B = Vdispl • ρliq • g It will seem as if the object weighs less, since the net downward force on it will be its weight due to gravity minus the upward buoyant force. w = weight of the object in air w΄ = “apparent weight” of the object in liquid (2) APPARENT WEIGHT w΄ = w - B (B) Full Immersion of a Solid A solid object of greater density than the density of liquid will sink in the liquid. In this case, a convenient way to find B is to compare its weight and its apparent weight: w = weight of the object in air w΄ = “apparent weight” of the object in liquid 62 (3) BUOYANT FORCE B = w - w΄ Experiment 13 For a fully submerged solid Vdispl equals the entire volume. From (1) and (3) a useful formula (4) follows: ρliq = density of the liquid. ρsol = density of the solid. M = w/g = mass of the solid in air. M΄ = w΄/g = “apparent mass” of the solid. (Note V = M / ρsol) APPARENT MASS RELATION (4) M • ρliq = (M - M΄) • ρsol C) Floating (Partially Submerged) Solid Object If a homogeneous solid object floats on the surface of a liquid then the buoyant force balances the weight. Then M’ = 0 and we have B = w. Then from this fact and (1) follows (5): FLOATING SOLID (5) Vsub = Vdispl = the fraction submerged V = entire volume of the solid. Vsub = ρsol V ρliq Procedure Part I: Density of a Pink Liquid The Laboratory Balance The type of balance you will use in this experiment, shown in Figure 1, is much more accurate than the equal arm balance. It also has a provision to measure the apparent masses of fully immersed solid objects: The masses are hung directly from the balance with a string while a beaker holding a liquid is supported from below. For the purposes of this experiment all masses must be measured to the accuracy of 0.1 gm. 63 Experiment 13 (a) Make sure that all your specimens are clean and dry. Measure masses M1, M2, and M3 of the three cylindrical specimens given, with the accuracy of 0.1 gm. Measure their lengths L and their diameters D with your calipers to 0.01 inch accuracy. Record these data on your data sheet. (b) Fill a beaker with sufficient amount of pink liquid, and suspend each one of your cylinders so that they are fully immersed (but not touching the bottom!). Measure and record their apparent masses M1, M2, and M3. Dry the cylinders. Retain the beaker with pink liquid for future use. NOTE: DO NOT PROCEED UNTIL YOUR LAB INSTRUCTOR checks your DATA. Procedure Part II: Density of an Irregular Solid Object (c) Record the mass M of your rock specimen. (d) Fill your other beaker with sufficient amount of blue liquid and suspend this specimen in it. Measure and record its apparent mass as M΄blue. The density of blue liquid is 1.26 gm/cm3; record it. NOTE: Return the blue liquid to its original bottle (do not discard it down the drain!). Rinse both your specimen and the beaker (use soap if necessary) and wipe them thoroughly with a paper towel. (e) Refill the (rinsed) beaker with tap water and proceed as in (d). Record the apparent mass of the rock as M΄water. (f) Wipe the rock again and suspend it in your (retained) beaker containing pink liquid. Record the apparent mass as M΄pink. (g) Return the pink liquid to its original bottle. Rinse and dry the rock specimen and both beakers. Procedure Part III: Density of a Floating Solid Object (h) Record the full length of the rod l in the same artificial units. Fill the graduated cylinder with tap water to some ¾ of its capacity. Slowly, lower the wooden rod until it floats freely. Make sure that the water level and the bottom of the rod are both within the scale, which is to be read in the artificial units of ml as length. Using the graduated cylinder’s scale, record: x1 = the level of the bottom of floating rod. x2 = the level of the water surface. 64 Experiment 13 BEFORE YOU LEAVE THE LAB: Make sure that all specimens and their boxes are clean and dry. Also, leave your station clean and dry. Discard all rubbish. Lab Report Part I: Density of a Liquid (1) For your cylindrical specimens construct Table 1, as shown. Enter your data. Calculate all required values accurate to three significant digits. The volume V of a cylinder is given by V = r2 L. TABLE 1. DENSITIES of CYLINDERS. Specimen D L V M ρsolid 3 # (cm) (cm) (cm ) (gm) (gm/cm3) 1 2 3 2) Set up Table 2 as shown. Fill it out with the same three significant digit accuracy. TABLE 2. DENSITY of the PINK LIQUID Specimen # 1 2 3 M M΄ ρsolid ρliq(PINK) Average < ρliq> Estimate the quality of your result by displaying the quantity: δPink = Max.Abs.Deviation AVE <ρliq> x 100% 65 ABSOLUTE DEVIATION in ρliq Experiment 13 Part II: Density of an Irregular Solid Object 3) For your Rock sample (quote its code number), use your average for the density of pink liquid and complete Table 3, as shown. Use three digit accuracy. Liquid Blue Water Pink TABLE 3. DENSITY of a ROCK SAMPLE ρliquid ρrock M M΄ M-M΄ 3 (g/cm ) ABSOLUTE DEVIATION in ρrock 1.26 1.00 (From Part I) Average <ρrock> Estimate the quality of your result by displaying the quantity: δrock = Max.Abs.Deviation AVE <ρrock> x 100% Part III. Density of a Floating Object 4) Answer Question #1: Explain why you may replace the left hand side of formula (5) by the ratio x2 – x1 no matter what were the units of length (even if “artificial”). l 5) Calculate and display the density of your wooden rod in gm/cm3 using this ratio. Part IV: Overall Review of Archimedes’ Principle 6) Answer these questions: Q #2: Suppose a solid object is fully immersed in a liquid, but B is larger than w. Is this possible at all? If yes, then: a) Does Archimedes’ Principle apply here at all? b) What happens to the object in this situation? Q #3: It is a well known fact that swimming is easier in the sea than in a freshwater lake. Does this relate to buoyant force or to some completely unrelated reasons? Clarify your statement. Q #4: Does Archimedes’ Principle apply for an object of non-uniform density? Whether your reply is “yes” or “no”, give your reasons. 66