#2 GROSS BRAIN AND SPINAL CORD
advertisement

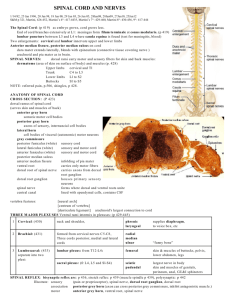
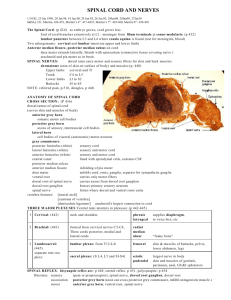
#2 GROSS BRAIN AND SPINAL CORD A. Medial Surface of Brain. Sagittally section the whole brain with a brain knife as close to the midline as possible. 1. The structures described in the following sections can be seen on the medial surface of the sagittally sectioned brain (H2-29). The photographs of the medial surface of the brain stem (H2-30) will be helpful in locating these structures. They also are depicted in the brain stem diagram (ND2) and on the next page. Begin at the caudal end and observe the relationship of the fourth ventricle (H2-30) to the medulla, pons and cerebellum. For the floor of the fourth ventricle refer to the brain stem. 2. The dorsal surface of the pons (H2-34) forms the rostral portion of the floor of the fourth ventricle. The lateral boundaries of the rostral portion of the fourth ventricle are the superior cerebellar peduncles (H2-34), comprised mainly of efferent fibers connecting the cerebellum with the mesencephalon and higher centers. The thin roof of the fourth ventricle is the superior medullary velum (H2-34, labeled anterior) which lies between the superior cerebellar peduncles. The floor of the fourth ventricle (ND99) is depicted diagrammatically in this guide. 3. The roof of the fourth ventricle is formed by the cerebellum and superior and inferior medullary vela (H2-30), but only the anterior (superior) is labeled. The inferior medullary velum is continuous with the posterior choroid plexus (H230). The inferior velum is perforated by three apertures (the two lateral foramina of Luschka and the medial foramen of Magendie) communicating with the subarachnoid space. These apertures are usually not demonstrable. In the floor of the fourth ventricle (rhomboid fossa) (H2-34) note the median sulcus (H234) and the sulcus limitans (H2-34). Look for the trochlear nerve (H2-24) emerging from the superior medullary velum. It is the only cranial nerve which exits dorsally from the brain. Because of its fragility, it is sometimes missing. 4. Next, on the half brain identify the cerebral aqueduct (H2-30) in the mesencephalon. It is a small channel connecting the fourth ventricle with the third ventricle; the latter is rostrally situated in the diencephalon. The part of the mesencephalon lying dorsal to the cerebral aqueduct is the tectal plate (H2-30, which means roof), which is the combination of the superior and inferior colliculi (H2-30), whereas the portion anterior to the aqueduct is the tegmentum (H2-30). 5. Observe the medial surface of the diencephalon which can be seen because it provides the boundaries for the slit-like third ventricle. Identify the fornix (H2-30) on the medial surface of your specimen. Look for a small opening beneath the rostral end of the fornix. This is the interventricular foramen (of Monro) (H230). Insert a probe in this foramen. This is the communication between the third ventricle and the lateral ventricles located in the cerebral hemispheres. B. 6. The Thalamus. The thalami (dorsal thalami) (H2-30) are oval masses, one on each side which form the lateral walls of much of the third ventricle. An interthalamic adhesion, massa intermedia (H2-30) may or may not be present. The thalami contain nuclei in the sensory pathways to the cerebral cortex. 7. The Epithalamus (Caudal, dorsal midline structures). The only feature of the epithalamus we will study is the pineal gland (H2-30). The pineal can also be found on the brain stems. 8. The Hypothalamus. The ventral portion of the diencephalon, hypothalamus (H230), includes, (a) the mammillary bodies (H2-30), which have important limbic system connections; (b) the tuber cinereum, to which the infundibulum (H2-30) of the pituitary gland is attached; and (c) the optic chiasm (H2-30). Spinal Cord Much of the basic anatomy of the spinal cord has been studied previously in Core I. This review is desirable because certain structural features have special functions. A videotape of a demonstration of the spinal cord is available for study. Several models and specimens of spinal cord are also in the laboratory. 1. 2. The Meninges a. The dura of the spinal cord is not fused to the surrounding bone (as in the brain) but is separated from bone by the loose connective tissue of the epidural space. For this reason the dura still surrounds the spinal cord which is demonstrated. Recall the anatomy of the meninges and the cord within the veterbral canal. The cord extends in adults to the lower border of L1 whereas the dural sac and arachnoid extend to S2. In newborn children the spinal cord occupies more of the vertebral canal, but the vertebral canal elongates more than the cord during maturation, producing the adult condition. Lumbar punctures are commonly performed for a variety of diagnostic purposes. Use is made of the presence of a lumbar CSF cistern and the protection afforded dorsal and ventral roots of the cauda equina by CSF. Could a subdural vs. subarachnoid hemorrhage be distinguished by a lumbar puncture? The particular relationships between the meninges and peripheral nerves are important in a variety of approaches for administration of anesthetic agents. b. Denticulate ligaments (H2-1), consisting of pia, are lateral stabilizers of the cord. They can be used as important landmarks for identifying the position of certain tracts within the spinal cord. Gross topography of the spinal cord (H2-1, H2-2, H2-4) a. On a spinal cord find the anterior median fissure (H2-2). This is a deep fissure on the ventral surface of the cord. Find the cervical enlargement and the lumbar enlargement. What is the reason for these enlargements? Locate the conus medullaris (H2-4) and the filum terminale (H2-4). Find a shallow groove, the posterior median sulcus (H2-2), on the dorsal side of the cord. On each side find the shallow anterolateral sulcus from which emerge the filaments of the ventral (motor) roots (H2-2) and posterolateral sulci for emerging dorsal (sensory) roots (H2-2). Note that the anterior median fissure, the posterior median sulcus, the two anterolateral sulci and the two posterolateral sulci extend the length of the cord. Root filaments that form the accessory nerve emerge from the lateral aspect of the upper cervical cord. Note that the root filaments (dorsal and ventral) form a dorsal root and a ventral root of the spinal nerve. A segment of the spinal cord is defined as the area which gives rise to the rootlets that combine to form a numbered spinal nerve. Review the approximate position of the cord segments in reference to vertebral levels. It should be emphasized that in neuroanatomy when reference is made to segments such as C5, T10, L1, spinal cord segments are being referred to, not the vertebral level at which the given peripheral nerve emerges. Transverse sections of the spinal cord (H5-1, H5-2, H5-3, H5-4 & H5-5) demonstrate the centrally located AH@ shaped gray matter (cell bodies) consisting of the posterior (dorsal) horns, an area of intermediate gray matter, and two anterior (ventral) horns. The posterior funiculus is the white matter (axons) between the two posterior horns whereas the anterior funiculus occurs between the two anterior horns. The two lateral funiculi occur between the anterior and posterior horns. These features are shown on the cut upper end of the specimen in the demonstration. 3. The blood supply (H2-3) The blood vessels on the lab specimens are difficult to see. Usually the veins can be visualized. a. As has been pointed out, the single anterior spinal artery (H2-21) and paired posterior spinal arteries (H2-21) arise from the vertebral arteries and proceed down the spinal cord. The posterior spinal arteries may arise from the vertebral or the posterior inferior cerebellar a. The anterior spinal artery lies along the anterior median fissure and supplies the ventral and much of the lateral funiculus. Additionally, the anterior portion of the gray matter of the cord is supplied by this artery. The anterior spinal artery supplies nervous tissue via sulcal arteries which alternate in supplying right and left sides of the spinal cord. The dorsal funiculus, dorsal portion of the lateral funiculi, and posterior part of the posterior horn receive blood from the posterior spinal arteries. The posterior spinal arteries (H2-3) lie parallel to the posterolateral sulci. Both the anterior and posterior spinal arteries are reinforced along their routes by some radicular branches (H2-3) from vertebrals, ascending cervicals, intercostals, and lumbar arteries, but they don=t exist at all levels as the spinal arteries diagram (H5-6) implies. b. The blood supply to certain levels of the cord may be easily compromised especially in areas of transition where blood is derived from two sources. For example, levels T1 through T4 cannot be maintained without the integrity of the intercostal vessels and their radicular branches. Another vulnerable level is L1. In the absence of adequate blood supply, necrosis will occur and the resulting lesion may resemble a transection of the cord at that level. These areas are of great concern to both neurologists and physicians in several surgical specialties. c. 4. The venous drainage of the spinal cord parallels the arterial supply with the addition of a midline posterior spinal vein. The two veins paralleling the posterior spinal arteries are the posterolateral spinal veins. The single anterior spinal vein courses with the anterior spinal artery. The spinal veins are drained by many anterior and posterior radicular veins which then empty into a venous plexus in the epidural space. Blood from this plexus passes through channels in the intervertebral foramina to the external vertebral plexus which is drained by vertebral, intercostal, and lumbar veins. The microscopic anatomy of the spinal cord a. Study spinal cord slides which show sacral (T1A), lumbar (T1B), thoracic (T1C) and cervical (T1D) levels of the spinal cord. Compare with sections from Haines of sacral (H5-1), lumbar (H5-2), thoracic (H53), lower cervical (H5-4) and upper cervical (H5-5). b. Re-identify in sections the topographical landmarks of sulci and fissures referred to in B on the previous page. c. Distinguish between the gray and white matter. Compare the relative amounts of each in the three cord levels presented by the sections. Why is there relatively little gray matter in the thoracic level compared with the other two? Why is there progressively more white matter as one ascends the cord as noted in comparing the volume of lumbar to cervical, for example? d. Is there any evidence of white matter (myelinated fibers) within the gray? Ex., anterior white commissure (H5-4). This will be significant later in studying the mode of cross-over of some of the sensory fibers. e. The nerve cells within the spinal cord should next be studied. The student should distinguish between anterior (ventral), intermediate, and dorsal horn cells. Each of these groups B sometimes called columns because they form a more-or-less continuous column longitudinally throughout the cord B have discrete functions. f. Thus, the intermediolateral cell column, which in this case is limited to thoracic and upper lumbar levels of the cord, represents the cells of origin of the preganglionic axonal fibers of the sympathetic nervous system. It has its counterpart in the sacral region but the fibers have a different pattern of termination and make up a component of the parasympathetic system. g. Nerve cells of like structure (largely a similar Nissl pattern) and like function (i.e., receive fibers from a common source) distribute their axons to a common destination. Those which are important will be specifically identified as the fiber systems are studied. Still, the student should appreciate the fact that it is possible to identify within the cord, on the basis of structure, discrete groups of neurons (cell bodies) which for example innervate the diaphragm (phrenic), are dorsal spino-cerebellar fibers (nucleus dorsalis), or are neurons projecting to specific muscles, such as the gastrocnemius, biceps. This will become far more significant later in studying the brain stem where very discrete nuclei have specific functions (e.g., oculomotor, facial, vagus). In summary, for the cord, dorsal horn nuclei are sensory, receptory and association; intermediolateral are autonomic (smooth muscle, cardiac muscle and sweat glands); and ventral are somatic motor (skeletal muscle). 5. 6. Study slides NH1, NH2, NH3, NH4, & NH5 to identify cells of the spinal cord as well as ganglia of the peripheral nervous system. a. Spinal Cord Cross Section (NH1). The anterior (or ventral) surface of this spinal cord is toward the bottom of this slide. Identify the gray and white matter. Locate the dorsal, ventral, and lateral funiculi. Note the large spinal ganglion adjacent to the cord. b. Anterior Horn Cell (NH2). This is a high power view of a large, motor neuron found in the anterior (or ventral) horn of the gray matter of the spinal cord. Identify the pale nucleus and deeply stained nucleolus. Nissl substance can be seen in the cytoplasm of this cell. Note the numerous processes of this multipolar cell. It does not appear that this section includes an axon; there is no obvious axon hillock. Surrounding the motor cell are many darkly stained nuclei which are nuclei of neuroglial cells. The cytoplasm of these cells does not stain unless special preparations are made. c. Spinal Ganglion Cells (NH3). This photograph is of a section through a dorsal root ganglion. Note the large, unipolar cell bodies. What may appear to be axon hillocks are probably shrinkage artifacts. How does the Nissl substance seen here differ from that of the motor cell? Identify the satellite cells surrounding the cell bodies. Many nuclei of connective cells can be seen between the cell bodies. Notice the bundle of myelinated fibers cut in cross section at the right of this slide. d. Autonomic Ganglion Cells (NH4). This is a low power photograph of a section through a sympathetic chain ganglion illustrating the morphology of the cell bodies that give rise to postganglionic fibers. Note the eccentrically placed nuclei of these multipolar cells. Satellite cells, although present, are not nearly as prominent around the cell bodies as those seen in the previous slide. Large bundles of myelinated fibers are not evident in autonomic ganglia. e. Autonomic Ganglion Cells (NH5). This is a higher power view of autonomic cell bodies. Histology of Peripheral Nerves Now turn your attention to the Awhite matter@ of the cord on the spinal cord slides. These areas are made up of (for the most part) axons of nerve cells from the gray matter or spinal ganglia which extend some distance in the CNS. Fibers from similar origin that will terminate on a discrete target are bundled together in what are referred to as tracts or fasciculi. However, it is not necessarily the case that all fibers within a tract have the same function. a. Cross Section of Myelinated Nerve (NH12). This slide shows many myelinated nerve processes bound together by connective tissues. In cross section the myelin resembles a doughnut and the nerve process, or axon, occupies the hole in the doughnut. The connective tissue between adjacent myelinated processes is the endoneurium. Nuclei seen within this fascicle are the nuclei of Schwann cells. b. Longitudinal Section of Myelinated Nerve (NH13). Identify the pink stained myelinated fibers on this slide. What are the elongated purple nuclei? c. Nerve Cross Section (NH14). This is a cross section of an entire nerve such as you have dissected out in the Gross Anatomy Laboratory. Surrounding the entire nerve is the epineurium a connective tissue sheath that will hold sutures. d. Vein, Artery and Nerve (NH15). Arteries, veins and nerves are frequently found together as indicated on this slide. The vein is a flattened tubular structure on the right whereas the artery has a fairly thick muscular wall. e. Nerve, Cross Section (NH16). This tissue is stained with osmic acid which stains the myelin black. Axons can be seen in some of the myelin sheaths. Identify the perineurium. f. Teased Myelinated Fibers (NH17). This slide is also stained with osmic acid to demonstrate myelin. Note the node of Ranvier at the arrow. To the left of the node a number of funnel-shaped Schmidt-Lanterman clefts can be seen extending in toward the axon. g. Oculomotor Nerve (NH18). Note the heavily myelinated fibers in this nerve. Axons can be seen in the center of the myelin sheaths. Surrounding the myelin is a very thin membrane composed of Schwann cell membranes. Can you identify Schwann cell nuclei? h. Choroid Plexus (NH37). This is a low power photomicrograph of choroid plexus taken from the roof of the fourth ventricle. Notice the proximity of a large artery to the villous like processes of the choroid plexus projecting into the ventricle. i. Choroid Plexus (NH38). This is a high power photomicrograph of choroid plexus. Notice the epithelial covering. These cells are joined by tight junctions. There is a capillary in the core of the process. Most of the real study of neuroanatomy concerns the location of nuclei, origin of nerve fibers, or fasciculi and their course to the next level of synapse. These will be studied through the course as the sensory and motor systems are covered. The material on the next three pages concerning fiber tracts in the central nervous system should be read before the next laboratory session. C. Fiber Tracts of the Central Nervous System 1. A study of fiber tracts and pathways within the central nervous system is best accomplished by use of selected sections taken at various levels through this system. One definition of a fasciculus is a nerve fiber tract having a common origin and termination and frequently a common function. The names of many of the fasciculi usually indicate the origin and termination of the nerve processes composing the fasciculi. A good example of this system of nomenclature is the spinothalamic tract. The cell bodies of the axons making up this tract are found in the spinal cord and these axons terminate in the thalamus. The cell bodies of axons in the corticospinal tract are located in the cerebral cortex and terminate in the spinal cord. Generally speaking, ascending tracts (spinothalamic) are sensory pathways whereas motor tracts (corticospinal) descend. 2. You have probably noticed that in studying the white matter of the spinal cord grossly and microscopically, it is impossible to identify any specific tract. In the usual preparations of this material, the white matter looks fairly homogeneous throughout. If you examine figures in your text and the photographs in your neuro atlas you will observe that most of the tracts and pathways that are an integral part of neuroanatomy have been precisely located. A knowledge of these localities is fundamental to clinical neurology. Several methods have been used in localizing these various fasciculi. 3. You will learn in a later lecture that, if a nerve cell body is destroyed or an axon is cut, the fiber distal to the site of the lesion will degenerate. This is anterograde degeneration. The myelin sheath surrounding a degenerating nerve fiber can be selectively stained with osmic acid (the Marchi method) while the surrounding normal fibers are unstained. The pathways of large groups of axons, such as the corticospinal tract, were originally described by observing patterns of degeneration in autopsy material from patients with localized lesions. Although much information was obtained with this approach, it was not sufficient to delineate the smaller and shorter projections which constitute most of the pathways within the CNS. Most our knowledge of neuroanatomy is derived from animal studies in which very small amounts of tracer substances or viruses are injected into precise regions of the CNS using stereotaxic instruments. After sacrificing the animal, various methods are used to determine both projections away from the site of injection as well as the sources of input to the region of injections. 4. During embryonic development all nerve tracts do not myelinate at the same time. The rate of myelination of different tracts exhibits much variation. In general, the phylogenetically older tracts myelinate first and are usually more centrally located, such as the vestibulospinal tract which becomes myelinated during the sixth fetal month. Phylogenetically newer tracts myelinate later on in development and occupy a more peripheral position in the central nervous system. An extreme example of this is seen in the corticospinal tract which does not begin to myelinate (or function) to any great extent until one or two years after birth. Selective staining of myelin is also a help in localizing specific tracts during development. D. 5. Much of the remainder of the laboratory portion of this course will be devoted to a study of some of the major fiber pathways of the CNS. Many of these pathways involve several neurons functioning in a network and you will be expected to know not only the pathway for a particular functional modality, but also, the nuclei associated with these tracts. Rarely will a pathway remain ipsilateral, or on the same side of the body. Most tracts will cross to the contralateral side and knowing the exact area of decussation of these fibers is important in working out neurological problems. Structures associated with, and adjacent to, specific tracts are significant as a Apure@ lesion of one specific pathway is seldom, if ever, seen. Tracts frequently change their position as they course through the CNS. By means of a study of cross sections of the brain stem coupled with your knowledge of gross topography you will develop a three dimensional concept of the CNS. 6. A convenient method of sorting out fiber pathways is to construct diagrams of them. For this reason, you will find a set of unlabeled outline drawings of selected sections from your slide boxes just before the next laboratory exercise. You are encouraged to utilize these diagrams for reconstructions of fiber pathways. You may want to use different colors to represent pathways which can be added in subsequent sessions. 7. The first pathways to be studied are the sensory pathways which arise from afferent peripheral nerve fibers bringing somatosensory information into the CNS. 8. For these pathways it will be necessary to know not only the pathway to the conscious level, but also, the reflex pathways that are involved. As a clinician, much of your neurological testing will be for the proper functioning of these arcs. You will be testing the sensory neuron that brings information to the CNS, the connections made by association neurons (if any), and the effector unit or muscle or gland that is stimulated. Brain Stem Slides 1. In the succeeding exercises, continual reference will be made to the set of brainstem slides that has been issued to your team. The collection of transverse sections was provided by Dr. Howard Meineke, Professor of Anatomy, University of Cincinnati, who received them from Dr. Alphonse Vonderahe, neurologist and neuroanatomist at the University of Cincinnati. The sagittal sections date back to the 1920's and were probably made here at UNMC by Dr. W.A. Willard. Only representative sections have been chosen for use in the laboratory exercises and these should be adequate to give you a good concept of the basic internal anatomy of the CNS. The sections are stained by the Pal-Weigert technique, which stains myelinated fibers blue to black. Unmyelinated areas do not stain with this technique. Thus, relatively unstained areas represent nuclei within the central nervous system. Occasionally, it will be possible to observe the bluishblack myelinated fibers coursing through unstained nuclear areas. As a result of this staining procedure, it is possible to distinguish fiber tracts from nuclei. Neuropathologists make use of this technique to determine pathological absence of myelin for affected fiber tracts. 2. Neuroanatomists have for centuries viewed transverse sections of the brain stem with the ventral aspect at the bottom of the page and the dorsal aspect at the top. All atlases and texts have used this convention. Unfortunately that is exactly opposite to the convention adopted when CT scans and MRI came into vogue. As you recall, for scans the body is to be viewed as if you are standing at the feet of a supine individual looking toward the head. Neuro texts and atlases are not likely to change soon, although Dr. Haines has made such a proposal. Just keep in mind that the scans you will see will look upside down to you. 3. You will remember from your examination of the gross features of the brain stem that it is curved as it is followed from the medulla through the thalamus. It is therefore very difficult to obtain true transverse sections of all parts of the brain stem. Some sections will show parts of two different aspects of the brain stem. The sections shown in the Haines atlas will be similar to those in your set but not identical because the planes of the sections in two sets are seldom the same. By using the diagrams on the first page of the atlas that you were loaned, you can determine approximately where the transverse sections and sagittal sections in your collection were made. Comparison of transverse and sagittal sections may also be helpful in determining more precisely what was cut in the transverse section. 4. The labeled photographs of the slides in the neuro atlas will be very helpful as you attempt to identify structures on the slides in the laboratory. Most important structures have been identified on one or several sections, but not every structure has been labeled in every section. These slides may also be viewed on the intranet. Legends for the thalamic nuclei: A = anterior Int. Med. Lam. = internal medullary lamina Int. Lam. = intralaminar nuclei MD = dorsomedial CD = centromedian P = pulvinar MG = medial geniculate LG = lateral geniculate VPM = ventral posterior medial VPL = ventral posterior lateral VL = ventral lateral VA = ventral anterior LP = lateral posterior LD = lateral dorsal