ideal gas law - Mr. Fischer.com
advertisement
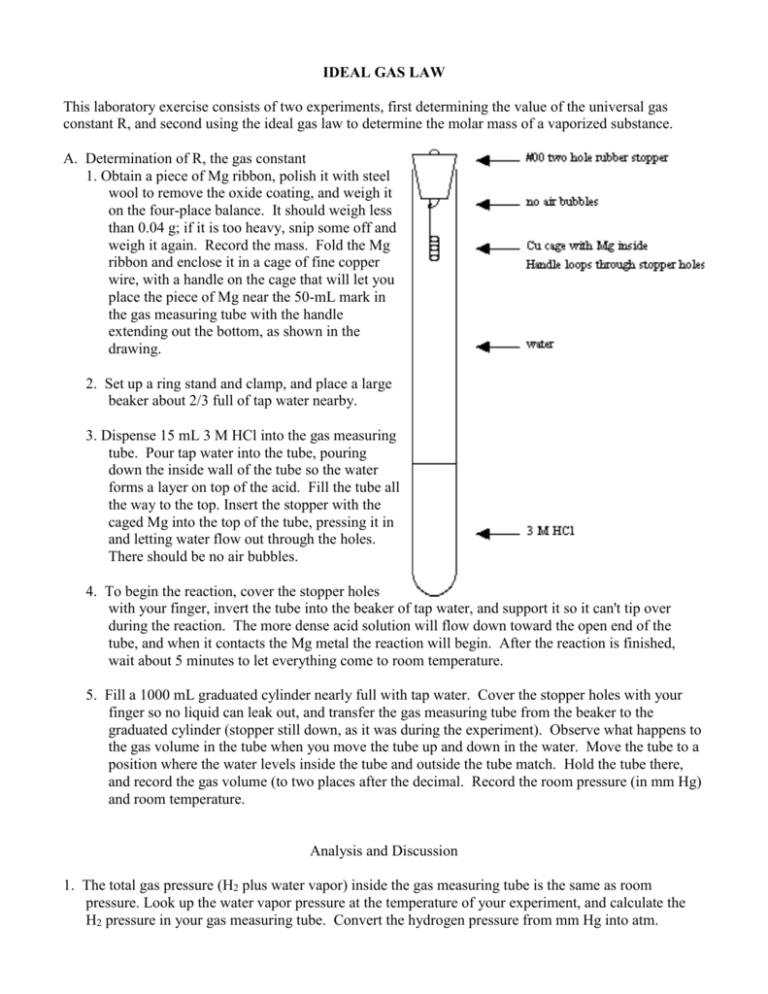
IDEAL GAS LAW This laboratory exercise consists of two experiments, first determining the value of the universal gas constant R, and second using the ideal gas law to determine the molar mass of a vaporized substance. A. Determination of R, the gas constant 1. Obtain a piece of Mg ribbon, polish it with steel wool to remove the oxide coating, and weigh it on the four-place balance. It should weigh less than 0.04 g; if it is too heavy, snip some off and weigh it again. Record the mass. Fold the Mg ribbon and enclose it in a cage of fine copper wire, with a handle on the cage that will let you place the piece of Mg near the 50-mL mark in the gas measuring tube with the handle extending out the bottom, as shown in the drawing. 2. Set up a ring stand and clamp, and place a large beaker about 2/3 full of tap water nearby. 3. Dispense 15 mL 3 M HCl into the gas measuring tube. Pour tap water into the tube, pouring down the inside wall of the tube so the water forms a layer on top of the acid. Fill the tube all the way to the top. Insert the stopper with the caged Mg into the top of the tube, pressing it in and letting water flow out through the holes. There should be no air bubbles. 4. To begin the reaction, cover the stopper holes with your finger, invert the tube into the beaker of tap water, and support it so it can't tip over during the reaction. The more dense acid solution will flow down toward the open end of the tube, and when it contacts the Mg metal the reaction will begin. After the reaction is finished, wait about 5 minutes to let everything come to room temperature. 5. Fill a 1000 mL graduated cylinder nearly full with tap water. Cover the stopper holes with your finger so no liquid can leak out, and transfer the gas measuring tube from the beaker to the graduated cylinder (stopper still down, as it was during the experiment). Observe what happens to the gas volume in the tube when you move the tube up and down in the water. Move the tube to a position where the water levels inside the tube and outside the tube match. Hold the tube there, and record the gas volume (to two places after the decimal. Record the room pressure (in mm Hg) and room temperature. Analysis and Discussion 1. The total gas pressure (H2 plus water vapor) inside the gas measuring tube is the same as room pressure. Look up the water vapor pressure at the temperature of your experiment, and calculate the H2 pressure in your gas measuring tube. Convert the hydrogen pressure from mm Hg into atm. 2. Convert the gas volume into liters and the temperature into Kelvins. 3. Calculate how many moles of H2 gas were produced in your reaction. The reaction is Mg (s) + 2 H1+ (aq) Mg2+ (aq) + H2 (g) 4. Use the ideal gas law to calculate the value of R, with appropriate units and sigfigs. 5. Calculate your % error and discuss sources of error in this experiment. 6. Why did the water level inside the tube change when you moved the tube up and down? Explain why it was necessary to equalize the water levels inside and outside the gas measuring tube before reading the gas volume. B. Molar mass of a volatile substance 1. Begin heating water in a large beaker (about ¾ full). Prepare an ice bath in another large beaker. Cut a piece of aluminum foil to form a cap when tightly crimped over the mouth of a round-bottom flask, and put a tiny pinhole into the center of the aluminum foil. Weigh the foil + flask. 2. Add about 0.5 mL of the unknown liquid to the flask, crimp the foil onto the mouth of the flask, and clamp the flask in the boiling water bath such that the flask is almost entirely submerged, but the foil is above water level. 3. As the liquid in the flask vaporizes, excess vapor escapes from the pinhole. Keep the flask in boiling water until it appears that all of the liquid has vaporized. Record the temperature of the boiling water bath. 4. Quickly cool the flask in an ice bath to condense the vapor. Dry the outside carefully (especially around the cap, where excess water may be trapped) and weigh the assembly. 5. Fill the flask completely with water and measure the volume of water in the flask with a large graduated cylinder. Record the barometric pressure. Analysis and Discussion Because the flask was an open system, the pressure of the vapor in the flask was equal to barometric pressure. The vapor volume was the same as the flask volume, and the vapor temperature was the same as the boiling water temperature. Combine these with the mass of trapped vapor to determine the molar mass of the liquid. Obtain the formula of the substance and calculate your percent error. There are many possibilities for error in this method, so don’t be surprised if your results are not very good. Identify at least two significant sources of error in this procedure that could explain your results.