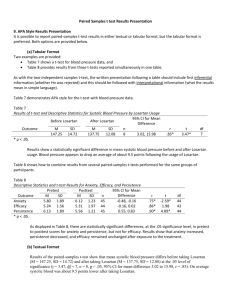
Antiproteinuric effects in nondiabetic hypertensives
advertisement

DRUGS OF TODAY Vol. 31, No. 7, 1995, pp. 463-498 LOSARTAN POTASSIUM (COZAAR™): A NONPEPTIDE ANTAGONIST OF ANGIOTENSIN II R.D. Smith, C.S. Sweet, A. Goldberg and P.B.M.W.M. Timmermans DuPont Merck Research Laboratories, Experimental Station, P.O. Box 80400, Wilmington, Delaware 19880-0400, and Merck & Co., Inc., 10 Century Parkway, Blue Bell, Pennsylvania 19422, USA CONTENTS Introduction ............................................................................................. Discovery of Losartan ........................................................................... Chemical strategy ................................................................................ Biological strategy................................................................................ Preclinical Pharmacology ........................................................................ Blockade of the physiological effects of angiotensin II .......................... Active metabolite (EXP-3174) .............................................................. Selectivity for the AT1 receptor subtype .............................................. Antihypertensive and antihypertrophic activity in experimental models of hypertension ................................................................... Angiotensin II as a growth factor acting via the AT1 receptor subtype .. Activity in models of cardiovascular disease ...................................... AT1 receptor antagonism in experimental models of heart failure........... Commentary . ...................................................................................... Clinical Experience with Losartan and Other AT1 -Selective Antagonists Clinical trials in normal subjects ........................................................... Clinical trials in salt-depleted volunteers .............................................. Uricosuric effect ................................................................................ Antihypertensive effects ....................................................................... Antihypertensive effects of EXP-3174.................................................. Antiproteinuric effects in nondiabetic hypertensives ............................ Concomitant administration with hydrochlorothiazide ........................... Safety and tolerability ........................................................................... Clinical trials in patients with heart failure ............................................. Conclusions ............................................................................................. References ............................................................................................. Introduction The discovery of losartan represents the culmination of an extensive search for an agent that would specifically block the pathological effects of angiotensin II (Ang II) at its receptor (1 -3). The rationale for this search was based on the role of Ang II in 463 465 465 466 467 467 468 469 470 471 471 474 475 475 475 478 479 479 480 480 480 480 481 482 484 the control of arterial blood pressure, the pathological role of Ang II in cardiovascular disease, and the therapeutic effectiveness of nonselective inhibitors of the renin-angiotensin system (RAS). Losartan is the first orally active, nonpeptide, long-acting and specific inhibitor of the RAS and represents: 1) a MEDICAMENTOS DE ACTUALIDAD 464 Fig. 1. The angiotensin system. (Reproduced with permission from Timmermans et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 1993, 45(2):205-251.) potentially important new therapeutic agent, 2) a powerful experimental tool for exploring the biological and pathological effects of Ang II, and 3) a prototype molecule for continued drug discovery. Losartan has already broadly stimulated interest in Ang II research and has been instrumental in the definition of Ang II receptor heterogeneity, the cloning of multiple Ang II receptors and subtypes, and in generating significant new insights into the biology of Ang II and how it interacts with its specific receptors. Presently, there are over 2000 publications describing the preclinical and early clinical experience with losartan (for reviews, see 4, 5). Angiotensin II is the primary mediator of the RAS and is involved in blood pressure control at the cellular, tissue and organ integration levels (5-7). Angiotensin II is an octapeptide that acts both as a circulating hormone and as a locally released and active "paracrine" agent (Fig. 1). Historically, the focus of RAS research and clinical discussion has been "renin" because methods were available to measure plasma renin activity (PRA) and it was not possible to routinely measure Ang II levels. The renin-sodium profile has even been identified as a risk factor for myocardial infarction in patients with hypertension (8). it is Ang II, however, that is the risk factor for and mediator of the cardiovascular pathology. Our current understanding of the pathological role of Ang II has evolved over a long period of time beginning with the clinical postulate by Bright, in 1836, that there was an association between renal disease and hypertension (Table I). The Goldblatt experiments (9) and the identification of the pressor hormone angiotensin were critical elements in the development of our current understanding. The synthesis of the pure peptide Ang II led to the investigation of the acute effects of angiotensin (vasoconstriction, aldosterone release) and the synthesis of peptide analogs of Ang II with varying degrees of agonist (Ang ll-like effects) and antagonist effects (18-20). One such peptide Ang II antagonist, saralasin, was evaluated in hypertensive patients and was shown to significantly lower blood pressure in patients with high PRA (21). This was the first important evidence that blocking Ang II resulted in antihypertensive effects in patients with essential hypertension. The peptide nature of saralasin meant that it was short-acting and had to be administered by constant infusion. Furthermore, as a close analog of Ang II, it retained significant partial agonist activity (22). It was evident early in clinical trials that saralasin significantly increased blood pressure in a significant proportion of low-renin patients (22), and was thus not suitable for general medical use. Our current understanding of the importance of the RAS in hypertension has also evolved from experience with other inhibitors such as -adrenoceptor antagonists, which have been shown to Table I: Discovery of angiotensin II. "Highlight" Reference • Postulated association between renal disease and hypertension, 1836 10 • Demonstrated pressor substance in renal extracts, 1898 11 • Renal artery constriction produced hypertension in dogs, 1934 9 • Independently showed that renin acted on plasma substrate to produce pressor hormone,1940 12,13 • "Hypertension" peptide isolated, 1956 14 • Angiotensin II formed by action of converting enzyme, 1956 15 • Independently synthesized the octapeptide "Ang II", 1957 Ang II = angiotensin II. 16,17 DRUGS OF TODAY VOL. 31,NO.7, 1995 inhibit renin release and are widely used antihypertensive agents (23). Since these agents have other pharmacological actions, the importance of this property of -biockers has been controversial (24, 25). However, some clinical studies have shown that the blood pressure-lowering effects of these agents are correlated to the pretreatment PRA (the higher the PRA, the greaterthe response), supporting a role for renin (Ang II) in hypertension (26). The most significant advance in the understanding of the role of Ang II in cardiovascular disease came with the development of inhibitors of Ang lI synthesis (for reviews, see 27-29). Angiotensin II is synthesized in a sequential fashion starting from angiotensinogen (30). The proteolytic enzyme renin (primarily from the kidney juxtagiomerular cells) cleaves the terminal 10-amino acid sequence to form the biologically inactive peptide Ang I. The proteolytic enzyme (located primarily on endothelial cells) angiotensin-converting enzyme (ACE) then cleaves Arg-Arg from the C-terminal of Ang I to form the mediator of the system Ang II. Since renin is ratelimiting for Ang II synthesis from angiotensinogen, renin inhibition was an early target for inhibiting the "angiotensin II system". Renin inhibitors modeled after pepstatin, and later after the active site of the renin enzyme, have been developed. These compounds are active in vitro and inhibit the RAS in experimental animals (for reviews, see 31-33). Although the oral efficacy of renin inhibitors has not been established, their effectiveness in lowering blood pressure supports the role of Ang II in the maintenance of blood pressure in hypertension. Blocking Ang II synthesis by inhibiting ACE has provided the strongest evidence that Ang II plays a role in cardiovascular disease. The efficacy of ACE inhibitors in the treatment of hypertension is now well established (27, 29, 34). Importantly, these compounds have been shown to decrease mortality in patients with congestive heart failure and have become a standard therapy for these patients (28, 35). In addition, the renal protective effects of ACE inhibitors in hypertensive patients with diabetes has been demonstrated, and it is therefore likely that inhibitors of Ang II synthesis will be protective in other forms of renal disease (36, 37). ACE inhibitors are, however, nonspecific inhibitors of Ang Il synthesis. ACE is also known as kininase II, and is thus responsible both for the synthesis of Ang II and for the catabolism and inactivation of the vasoactive peptide bradykinin. Bradykinin is a powerful vasodilating, analgesic and proinflammatory agent (38). Unlike renin, which is substrate-specific, ACE has several natural substrates, including enkephalin and substance P. The most widely studied nonspecific effect of ACE inhibitors has been 465 their effect on bradykinin (39). It remains controversial how important bradykinin potentiation is to the beneficial effects of ACE inhibitors in, e.g., regression of cardiac hypertrophy in coarctation hypertension in rats, pro (40), con (41), the vascular response to bailooon injury, pro (42), con (43, 44), or reduction in infarct size, pro (45), con (46). It is clear, however, that ACE inhibitors are not selective inhibitors and block Ang II synthesis while increasing tissue levels of bradykinin (47). Since the maximum blood pressure reductions with Ang II receptor blockade and ACE inhibition are comparable (48) and infusion of a bradykinin antagonist (Hoe-140) at the peak of antihypertensive response had no effect on losartan and little effect on the responses to the ACE inhibitors (49), it is likely that most of the blood pressurelowering effects of both classes of agents are due to blockade of Ang II or interruption of Ang II formation, respectively. Whether bradykinin exerts other beneficial effects remains largely speculative, but the undesirable effects of bradykinin have been extensively studied (38, 50). Bradykinin, when applied to isolated guinea pig tracheal preparations, stimulates C-fiber firing rate (38, 51), and thus may be implicated in the cough observed in some patients treated with ACE inhibitors (38). The collective clinical efficacy of inhibitors of the RAS provides strong evidence that Ang II is involved both in the maintenance of blood pressure and in the expression of the pathological changes that accompany hypertension in the heart and kidney, and possibly in other organ systems. Since it is known that Ang II elicits its biological effects by binding to specific sites on the effector organs (vascular smooth muscle, adrenal, kidneys) (6, 52), the most effective and specific way to block the effects of Ang II is to block the receptor site. Furthermore, Ang II can be synthesized from angiotensinogen by nonrenin pathways (e.g., tonin, chymotrypsin-like angiotensin-generating enzyme, tissue plasminogen activator) (53) or from Ang I by non-ACE or alternative pathways (e.g., chymase) (54). Receptor blockade may provide a more complete inhibition of the actions of Ang II than is possible by inhibiting ACE or renin. Based on this understanding of the "angiotensin system" and its role in cardiovascular disease, a new drug discovery program was undertaken to find a nonpeptide Ang II receptor antagonist (1-3). Discovery of Losartan Chemical strategy The medicinal chemistry that led to the discovery of losartan began with a focus on peptide analogs of Ang II, but was soon replaced by exploration of a 466 MEDICAMENTOS DE ACTUALIDAD Fig. 2. Chemical structures of some nonpeptide Ang II receptor antagonists illustrating the structural modifications of the initial lead compounds, S-8307 and S-8308, to EXP-7711, a more potent and orally active antagonist, through the key intermediates, EXP-6155 and EXP-6803. (Reproduced with permission from Timmermans et al. The discovery of a new class of highly specific nonpeptide angiotensin II receptor antagonists. Amer J Hypertension 1991, 4 (4, Pt. 2): 275S-281S. series of nonpeptide molecules described in patent literature as Ang II antagonists. These compounds, were described as hypotensive imidazole or hypotensive imidazole-5-acetic acid derivatives, and although no biological data were provided, they were reported to have Ang ll-antagonist effects (55). Examples (S-8307, S-8308) were synthesized and shown to have only very weak binding affinity for the Ang II receptor and displayed no significant blood pressure-lowering effects. However, they were specific and blocked the contractile response to Ang II while having no effect on the responses to norepinephrine or potassium chloride (56). A theoretical model was proposed whereby the carboxy imidazole mimicked the essential binding site of the carboxyterminal portion of the natural hormone Ang II. Chemical modifications were then focused on the phenylmethyl portion of the lead molecules (S-8307). The chemical strategy and the milestone compounds have been extensively reviewed (13). In brief, four compounds represent the extensive chemical synthetic effort (Fig. 2). These compounds have progressively increasing affinity for the Ang II receptor. The penultimate compound EXP-7711 represents the first molecule with significant oral antihypertensive activity. The chemical breakthrough came with the synthesis of the biphenyltetrazole molecule. The biphenyltetrazole represented completely novel chemistry and imparted high affinity and oral bioavailability to the candidate compound DuP-753, later designated losartan (USAN: losartan potassium). The chemical discovery of losartan has initiated an explosion of patents involving losartan as a molecular model and/or including the biphenyltetrazole as an important part of the lead compounds (57,58). Biological strategy The discovery of losartan was based on the need to show that a new molecule: 1) binds to the Ang II receptor with high affinity, 2) displays high specificity for the Ang II receptor, 3) blocks the contractile response of vascular smooth muscle to Ang II in vitro, 4) blocks the pressor responses to Ang II in vivo, 5) lowers blood pressure in renin-dependent (Ang Il-dependent) hypertension by both the intravenous (i.v.) and oral (p.o.) routes, and 6) is free of Ang ll-like agonist properties. Losartan was shown to have high affinity for Ang II receptors in competitive binding assays using membrane receptors prepared from homogenized tissues or in fixed tissues using autoradiography. The concentrations producing 50% inhibition (ICso) of Ang II binding range from 5 to 30 nM for losartan. In contrast, losartan (10 M) had no effect on other receptor ligands and growth factors (59). In more recent studies, inhibition of binding to thromboxane (60) and neurokinin NK3 (61) binding sites has been reported at concentrations of > 15 M (> 1000-fold DRUGS OF TODAY Vol. 31, No. 7, 1995 higher concentrations than those needed to inhibit Ang II). Blockade of the vascular smooth muscle response to Ang II in vitro using rabbit aortic strips established that losartan was a functional and competitive antagonist of Ang II receptors (48). Subsequent studies with other vascular tissues have confirmed the Ang ll-antagonist effect of losartan (62, 63). PD-123177, in contrast, has no effect on the vascular response to Ang II (48, 64). The specificity of losartan for the Ang II receptor was shown by the lack of effect on the contractile responses to norepinephrine or potassium chloride (48). The specificity of losartan was also confirmed by a study in isolated arteries and veins. In this study, the pA2 values (negative log of antagonist concentration required to double the agonist concentration) for losartan against Ang ll-induced contractile responses were 8.19-8.66, whereas the responses to norepinephrine, acetylcholine, bradykinin, desArg9-bradykinin, substance P, neurokinin A, neurokinin B or bombesin were unaffected (65). The inhibitory effect of losartan on the pressor response to Ang II in pithed rats showed that the blockade of Ang II receptors was also competitive in nature in vivo. Specificity was shown by the lack of effect on the pressor responses to vasopressin or norepinephrine (48). Losartan and its predecessors (Fig. 2) were initially evaluated for antihypertensive efficacy in renal hypertensive rats with renal artery ligation. EXP-7711 was the first compound that demonstrated significant oral antihypertensive activity (ED30= 11 mg/kg). Losartan was chosen after demonstrating potent antihypertensive effects by both the i.v. and p.o. routes (ED30 = 0.8 and 0.6 mg/kg, respectively) (66). Preclinical Pharmacology Blockade of the physiological effects of angiotensin II The basic preclinical pharmacology of losartan and other Ang II AT1-selective antagonists is outlined in Table II. Losartan blocks all of the well-known effects of Ang II, including constriction of vascular and nonvascular smooth muscle, aldosterona synthesis and release, drinking (in rodents), vasopressin release, feedback inhibition of renin release, and enhancement of norepinephrine release from sympathetic nerve endings (4,5). In isolated vascular tissue, losartan is a competitive antagonist of Ang II while having little effect on the contractile responses to norepinephrine or potassium. The IC50 values for losartan range from 19 to 50 nM and the pA2 values 467 Table II: Losartan preclinical pharmacology.a • Selectivity inhibits binding of Ang II to AT1 receptors in vitro Vascular smooth muscle Kidney Adrenal Heart Brain • Selectively inhibits responses to Ang II in vitro Vascular contraction Aldosterone synthesis/release Norepinephrine release from sympathetic nerves Cardiac myocyte "growth" Vascular smooth muscle cell "growth" • Selectively inhibits responses to Ang II in vivo Pressor response Aldosterone release Inhibition of renin release Cardiac/vascular hypertrophy Vasopressin release Drinking behavior • Lack of Ang ll-like agonist effects Isolated vascular and cardiac cells in vitro Normotensive and hypertensive animals in vivo • Lowers blood pressure (blocks endogenously released Ang II) Renal hypertensive rats (2-kidney, 1 clip) Spontaneously hypertensive rats Transgenic rats [TG(REN2)27] Reduced renal mass-induced hypertension Coldinduced hypertension Fructose dietinduced hypertension L-NAME induced hypertension a See Refs. 5, 80-82. from 8.2 to 8.7. Likewise, in vivo in pithed rats, losartan blocked the pressor response to exogenously administered Ang II in a competitive manner, shifting the dose-response curve to the right without altering the maximum response. Similar Ang ll-antagonist effects of losartan have been demonstrated in a number of animal species, including dogs (67, 68), guinea pigs (69), monkeys (70) and man (71), Angiotensin II stimulates aldosterone synthesis in isolated adrenocortical cells in vitro and the release of aldosterone In vivo, and losartan blocks both effects (72). In chronic studies in spontaneously hypertensive rats (SHR) and normotensive rats, losartan significantly reduced the plasma aldosterone levels (73, 74). The dipsogenic effects of Ang II can be readily demonstrated by central or peripheral administration in rats. In the initial study, losartan administered subcutaneously (s.c.) blocked the s.c. Ang II drinking 468 response, and when administered intracerebroventricuiarly (i.c.v.), it blocked the response to i.c.v. Ang II. Losartan given s.c. did not block the drinking response to i.c.v. Ang II, suggesting that losartan does not cross the blood-brain barrier (75). The drinking responses to Ang II have been blocked by losartan in several other species, including cows, rabbits and sheep (76). Conflicting data have been reported concerning the brain penetration of losartan, but in some cases inhibition of binding and functional antagonism have been demonstrated (77-79). Angiotensin II exerts a tonic inhibitory effect on renin release and blockade-of Ang II synthesis or of AT1 receptors increases circulating PRA. Losartan increases PRA in all species tested (e.g., rats, dogs, sheep, monkeys and man) (5). Likewise, Ang II levels rise and remain elevated throughout the duration of dosing. No development of tolerance to the antihypertensive effects of losartan has been observed, and no untoward effects due to the elevated circulating Ang II levels have been noted either in experimental animals or man (83). The regulation of the Ang II receptor does not seem to follow rises or falls in Ang II levels; infusion of Ang II increases mRNA for the AT1 receptor in adrenal but not in other tissues, whereas nephrectomy does not seem to change receptor number or affinity (84). Angiotensin II is involved in vasopressin release and this effect is blocked by losartan. The vasopressin released by i.c.v. Ang II was significantly reduced by losartan injected into the paraventricular nucleus (85). Losartan administered i.c.v. also blocked or reduced the vasopressin release induced by Ang II (86-88). There is some controversy as to whether i.c.v. PD-123319 has an effect on vasopressin release (87, 88). The release of norepinephrine from sympathetic nerve endings can be modulated by Ang II and this effect is blocked by losartan (or its active metabolite EXP-3174) (89, 90). Sympathetic nerve stimulationinduced release of norepinephrine from isolated cardiac and vascular tissue is enhanced by Ang II and is mediated by the AT1 receptor. It has been shown that atrial release of norepinephrine is suppressed in SHR and chronic treatment with losartan normalizes this response (91). In electrically stimulated human kidney cortical slices, Ang II produced a concentra- < tion-related increase in radio-labeled norepinephrine release, which was reduced by captopril and abolished by EXP-3174 (92). In vivo in renal hypertensive rats, losartan and lisinopril lowered blood pressure and increased sympathetic nerve activity less than the nonspecific vasodilator nitroprusside (93). The sympathetically mediated vasoconstriction of norepinephrine was also reduced or inhibited in other experimental preparations (94-96). MEDICAMENTOS DE ACTUALIDAD The principle evidence that losartan blocks the effects of endogenously released Ang II comes from its ability to lower blood pressure in Ang II (renin)dependerit hypertension in rats. In rats with elevated PRA, losartan displays a dose-related blood pressure-lowering effect both acutely and following chronic dosing (97-99). Losartan at 30 mg/kg for 8 weeks significantly decreased blood pressure and reduced ventricular hypertrophy in two-kidney, one-clip renal hypertensive rats, whereas similar treatment with the nonspecific vasodilator hydralazine did not (100). Other "high-renin" models of hypertension such as the transgenic rat harboring the REN-2 gene are also sensitive to the blood pressure-lowering effects of losartan (101). In contrast, losartan has little or no effect in normotensive animals or animals such as the Dahl salt-sensitive rat in which Ang II is suppressed (97). Some investigations have shown that chronic losartan treatment did have a significant blood pressure-lowering effect in normotensive animals, suggesting a role for Ang II in maintaining basal blood pressure levels (74, 102, 103). Normotensive animals can be made sensitive to Ang II system inhibitors by sodium depletion with diuretics or a low-sodium diet. In the sodium-depleted dog, losartan produces a dose-related decrease in blood pressure, whereas it has very little effect in the sodiumreplete dog (104). Similar findings have been found in man (105). Active metabolite (EXP-3174) Losartan is active by itself, as demonstrated both in vitro and in vivo, but in some species, including rats and man, it is also converted to an active metabolite, designated EXP-3174 (Fig. 3). EXP-3174 is the free carboxylic acid of losartan and displays the same pharmacological profile (106, 107). The metabolite is 10-40 times more potent than losartan in isolated vascular tissue. In pithed rats, EXP-3174 produces a nonparallel shift of the Ang II dose-response curve and decreases the maximum response, consistent with noncompetitive blockade. Since it is possible to displace EXP-3174 binding with excess unlabeled material (108) and pretreatment of isolated tissue with losartan converts the "noncompetitive" blockade of EXP-3174 to competitive blockade (63), it is likely that this reflects tight binding or slow release from the receptor. This is further supported by the finding that EXP-3174 was washed out of the tissue more slowly than losartan (63). Thus, in rats and man (data discussed below), "losartan treatment" is the net effect of AT1 receptor blockade with losartan and EXP-3174. In the dog, and presumably in guinea pigs and sheep, little of this metabolite is formed and losartan is rapidly 469 a Christen et al. (112). Fig. 3. Pharmacokinetic highlights of losartan and its active metabolite EXP-3174 in man. cleared as the glucuronide (109-111). This means that studies carried out in these species must use multiple-dose regimes or drug infusions to maintain receptor blockade. Selectivity for the AT1 receptor subtype The discovery of losartan and the concurrent discovery of two other series of compounds opened the door to our current concept of Ang II receptor heterogeneity. Although the existence of multiple Ang II receptor subtypes had been suggested by earlier findings (for review, see 5), it was not until losartan, PD-123177 and CGP-42112 became available that the existence of multiple Ang II receptors was confirmed and the complexity of Ang II receptor heterogeneity was realized (113,114). The basic observation was that losartan (or related compounds) inhibited all of the Ang II binding in receptor preparations from vascular smooth muscle, but in adrenal cortical receptor preparations 20-30% of the receptor sites were "resistant" to blockade. PD-123177 and CGP-42112 were found to inhibit these resistant sites and the concept of two receptor subtypes was reported by two independent laboratories (115,116). After a flurry of activity to subtype every species and tissue and to apply "novel" subtype designations, a standardized nomenclature was proposed (117). Accordingly, the losartan-sensitive site is the AT1 subtype and the AT2 site has affinity for the PD-123177 series of compounds and CGP-4211.2. Recently, this nomenclature has been extended to include the subsequently cloned rodent AT 1 subtypes and the nonmammalian receptors that do not fit the previous definition of AT1 or AT2 (118). It should be noted that saralasin and other peptide analogs of Ang II are "nonselective" and have high affinity for both Ang II binding sites. Likewise, since renin and AGE inhibitors block the synthesis of Ang Il and reduce the availability of Ang II to both receptor subtypes, they are "nonselective" inhibitors. Losartan is the prototype of an AT1-selective antagonist, so that if losartan inhibits a response it is by definition an AT1-mediated response (117). Virtually all of the known effects (functions) of Ang II are AT1-mediated, e.g., vasoconstriction, aldosterone release, drinking, inhibition of renin release, vasopressin release and enhancement of norepinephrine (4, 5). The early findings with losartan have now been confirmed by published studies with a number of new nonpeptide AT1-selective antagonists (62, 119, 120). Angiotensin II interacts with specific receptors on effector cells (e.g., smooth muscle cells or adrenal cortical cells) and elicits a series of intracellular events that lead to the characteristic response of that ceil (e.g., contraction or secretion). This hormone-synthesis-receptor-response coupling has been well studied for Ang II (121). Each functional step in this series of events is antagonized by losartan or related AT1-selective antagonist molecules, suggesting that they are AT1 receptor subtypemediated (Table Ill). This is confirmed by the lack of effect of PD-123177, PD-123319 or CGP-42112. It should be noted that CGP-42112 is a peptide and has been described as an agonist (118), but may act as an AT2 antagonist or an AT1 antagonist at high concentrations (122). MEDICAMENTOS DE ACTUALIDAD 470 Table III: AT1 -selective blockade of the Ang II receptor response coupling. Ang II "response" Receptor binding Antagonist L, S Reference 123 G-protein coupling Second messenger Intracellular response Cell/tissue/organ response Whole animal response GTP S -Adenylate cyclase - PLC - PLD L (not CGP or PD) L (not PD) L (not CGP) IP3 hydrolysis Ca2+ transient L (not CGP) L (not PD) 124,125 72 126 127 128, 129 130 Contraction L(notPD) 48 Secretion Transport Proliferation L, S (not PD) L (not PD) L (not PD) 131 132 133 Blood pressure L (not PD) 48 Renal function Drinking L, S (not PD?) L (not PD) 134, 135 48, 136 L = losartan; S = saralasin; CGP=CGP-42112; PD = PD-123177 or PD-123319; GTP S = guanosine thio-triphosphate; IP3 = inositol triphosphate; PLC = phospholipase C; PLD = phospholipase D. (Reproduced with permission from Timmermanns et a/., in press.) The role of the AT2 receptor is not well understood. PD-123177-like compounds do not lower blood pressure or block the vasoconstrictor effects of Ang II, so the functional role of this binding site in hypertension or blood pressure control remains to be determined (48,104). The AT2 receptor has been cloned and the structure is 32-34% homologous to the AT1 receptor. The AT2 receptor is not G-proteincoupled and it remains controversial how it is coupled to protein tyrosine phosphatase (137, 138). The abundance of AT2 sites in fetal and reproductive organs such as the uterus has suggested a growth or developmental role, but a functional response has not been determined (139, 140). The increased expression in rat skin wounds suggested a repair function, but AT2 sites were not found in rapidly dividing cell cultures (141). Studies from co-cultures of brain stem and hypothalamus from 1-day-old rats suggest that Ang II has an effect on a potassium current that is selectively blocked by PD-123177, but the meaning of this finding is unknown (142, 143). That the AT2 site has a growth-inhibitory role has been suggested by two reports. In the first, overexpression of the AT2 receptor at the site of balloon injury in rats suppressed neointimal formation (144). In this rat balloon injury model, AT1 receptor blockade also suppressed the neointimal response and addition of PD-123177 had no further effect (44,145, 146). In the pig, neither AT1 blockade nor the combination of AT 1 and AT2 blockade had any effect on the vascular response to balloon injury (147). It has recently been reported that Ang II acting through an AT2 site inhibits the proliferation of coronary endothelial cells cultured from SHR (148). If this is confirmed, it would mean that losartan and related AT1-selective antagonists would be antiproliferative by blocking Ang II directly at the AT1 receptor (the majority of effect) and indirectly through stimulation of the unblocked AT2 site by the elevated circulating Ang II levels. Preliminary data from balloon vascular injury in dogs suggest that AT1 receptor blockade inhibits neointimal formation, whereas ACE inhibition is ineffective. This is presumably due not to differences in effect on subtype, but rather to blockade of non-ACE-generated Ang II (149). Antihypertensive and antihypertrophic effects in experimental models of hypertension The efficacy of losartan in experimental models of hypertension provides the basis of the clinical trials in hypertensive patients and its subsequent marketing for the treatment of hypertension (5,150). The initial findings with AT1-selective blockade with losartan have now been confirmed with many other AT1 -selective antagonists, including candesartan (TCV-116) (62), irbesartan (SR-47436) (119) and valsartan (120). As described above, losartan has acute antihypertensive effects in renal hypertensive rats in which PRA (and presumably Ang II levels) are elevated (97). Rats infused with Ang II (30 mg/min) for 2 weeks develop hypertension and cardiac hypertrophy, which is blocked by losartan but not by enalapril. In contrast, lowering of blood pressure-with hydralazine does not block the hypertrophy (151). In DRUGS OF TODAY Vol. 31, No. 7, 1995 renal hypertensive rats (two-kidney, one-clip) dosed for 2 weeks, losartan lowered blood pressure and reduced both cardiac and vascular hypertrophy (98, 99). In rats made hypertensive by aortic constriction above the renal arteries, losartan, but not PD-123319, reduced cardiac hypertrophy and transforming growth factor-1, (TGF- 1) levels (152). Losartan and ramipril were comparable and both were superior to hydralazine in reducing cardiac hypertrophy in rats with ascending aorta constriction (153). Losartan has been extensively studied in SHR, which have low PRA (Ang II levels). In the initial study of losartan and captopril dosed for 14 days, both compounds produced comparable reductions in blood pressure (154). Importantly, after termination of treatment, the blood pressure slowly returned toward the pretreatment (vehicle) values, such that no rebound hypertension was observed. Ten-week treatment of 3-week-old SHR with either losartan or captopril significantly lowered blood pressure and resulted in longlasting reductions in cardiac and vascular hypertrophy (155). In these studies, the reductions in cardiovascular hypertrophy were assessed 17 weeks after the termination of treatment, suggesting that AT 1 selective blockade lowers blood pressure and affects the vascular and cardiac tissue changes associated with the development and/or maintenance of hypertension. Significant reductions in vascular and cardiac hypertrophy were observed 2 weeks after dosing with losartan, whereas comparable blood pressure reductions with hydralazine did not alter the cardiovascular hypertrophy (156). As expected, the acute antihypertensive effects of losartan are not bradyki-nin-dependent. In SHR, ramiprilat, captopril and losartan produced comparable reductions in arterial blood pressure. Infusion of the bradykinin antagonist Hoe-140 produced small reversals of the effects of the ACE inhibitors, but had no effect on the response to losartan (49). The antihypertensive effects of selective AT1 receptor blockade in SHR (young, old) are now well established, and the reduction in blood pressure is accompanied by decreases in cardiac and vascular hypertrophy (for review, see 82). Angiotensin II as a growth factor acting via the AT1 receptor subtype The growth-stimulatory effects of Ang II were first observed in 3T3 cells (157), and have subsequently been observed in a variety of cell types, including vascular smooth muscle cells, cardiac myocytes, mesangial cells, fibroblasts and endothelial cells (158, 159). Each of the cell types has important potential implications for cardiovascular hypertrophy and tissue remodeling. In vitro, Ang II stimulates the 471 expression of proto-oncogenes (e.g., c-fos), DNA synthesis (thymidine incorporation), protein synthesis or accumulation, and in some cases it increases cell number (Table IV). In each case, the "growth effects" of Ang II are blocked by losartan, but not by PD-123319 or CGP-42112 (see above). In vivo, the growth-stimulatory effect of Ang II is difficult to separate from the blood pressure effect. Angiotensin II infusion increases blood pressure and induces cardiac hypertrophy, and blocking the increase in blood ' pressure with hydralazine does not block the cardiac hypertrophy (151). Losartan, in contrast, blocks both responses, suggesting a pressure-independent effect of Ang II. In most studies in which losartan reduced or prevented cardiovascular hypertrophy, there was a significant reduction in blood pressure (99, 160). In Dahl salt-sensitive rats and strokeprone SHR, losartan has been shown to reduce ventricular hypertrophy while having much less effect on blood pressure, suggesting a dissociation between pressure and growth (161, 162). In newborn pigs, Ang II appears to have a trophic effect on heart weight, as the rapid increase in normal growth in heart weight is reduced by enalapril or losartan (163). As discussed above, the neointimal growth in response to balloon injury in normotensive rats and rabbits is blocked by losartan, suggesting that AT1 receptors are involved (for review, see 81). Activity in models of cardiovascular disease Hypertension is the primary indication for losartan and AT1selective antagonists. The efficacy of these molecules in experimental hypertension has been well documented (see above). In addition, losartan lowers blood pressure and/or exerts tissueprotective effects in models of renal failure, cardiac failure and stroke (Table V). Importantly, losartan, like ACE inhibitors, appears to increase survival in coronary artery-ligated "heart failure" rats (164), and in stroke-prone SHR (162) and Dahl salt-sensitive rats (161). The confirmation of these initial studies with losartan with other ATrselective antagonists points to the emerging concept that Ang II acts as a pathological factor to: 1) cause vasoconstriction, 2) induce tissue damage, and 3) decrease survival (Fig. 4). In the kidney, Ang II has multiple sites of action on afferent and efferent arterioles, proximal tubular cells, mesangial cells, JG cells, vascular endothelial cells, adipocytes and sympathetic neurons. The distribution and type (AT1 vs. AT2) of Ang II receptors appear to vary somewhat from species to species (192). In rat kidney, AT1 receptors predominate and are localized in the glomerulus, renal tubules and renal vasculature. In human kidney, the AT1 receptor predominates, although AT2 receptors are present in MEDICAMENTOS DE ACTUALIDAD 472 Table IV: The angiotensin II receptor subtype (or "growth" is AT1. Angiotensin response Species tissue or cell Proto-oncogene expression Rat hepatocytes Rat mesenteric artery Rat aortic smooth muscle cells Rat brain Rat heart fibroblasts Bovine adrenal cells Rat heart endothelial cells Growth factor expression (TGF-1, endothelin-1) Growth DNA synthesis (thymidine incorporation) Growth protein accumulation Growth heart weight Receptor subtype Reference AT1 AT1 AT1 AT1 AT1 AT1 165 166 167 168 169 170 AT1 171, 172 AT1 173, 174 AT1 AT1 AT1 AT1 175 131 176 177 AT1 178, 179 AT1 AT1 AT1 180 181 182 AT1 183 Rat aortic smooth muscle cells Mouse mesangialcells Bovine adrenal cortical cells Rat, human mesangial cells Human mesangial cells Rat cardiomyocytes Rat aortic smooth muscle ceils Rat aortic segments Rat mesangial cells Pig (newborn) heart TGF- 1 = transforming growth factor- 1. (Reproduced with permission from Smith and Timmermans. Human angiotensin receptor subtypes. Curr Opin Nephrol Hypertension 1994, 3: 112-122.) Table V: Efficacy of losartan in experimental models of cardiovascular diseasesa. Referencea "Disease" Model Finding Hypertension SHR, renal MAP, no rebound hypertension, SBP, Cardiac and vascular hypertrophy 154, 184 Renal failure Reduced renal mass Streptozotocin SBP, proteinuria Proteinuria, glomerular sclerosis, improved hernodynamics 185-187 Cardiac failure Aortocaval shunt Coronary ligation Cardiac hypertrophy LVEDP, cardiac hypertrophy, survival 164, 188-190 Stroke Stroke-prone SHR SBP, survival, cerebral infarction 162, 191 SHR = Spontaneously hypertensive rats; MAP = mean arterial pressure; SBP = systolic blood pressure; LVEDP = left ventricular end-diastolic pressure. aSee Ref. 5 for additional references. large preglomerular vessels in the renal cortex and tubulointerstitium (193). Angiotensin II appears to exert a complex modulatory role on normal basal renal function (194, 195), and under normal conditions, Ang II system inhibitors may have limited net effects (104, 196). However, when renal tissue is damaged by toxins or pressure load, or when the functional renal mass is reduced below that necessary to maintain normal sodium balance, the remaining JG cells are stimulated to release renin to increase circulating and tissue Ang II levels. Progressive reductions in glomerular filtration rate (GFR), sodium retention, proteinuria and glomerular sclerosis follow the rise in Ang II. Blockade of Ang II synthesis with ACE inhibitors has provided the best evidence that Ang II exerts a pathological effect in the kidney (197). The nonspecific actions of these compounds, e.g., bradykinin potentiation, however, complicate such interpretations. Since bradykinin is a potent vasodilator of afferent arterioles, ACE inhibitors, by blocking Ang II synthesis, may have a greater and potentially adverse effect on GFR (198, 199). AT1 receptor blockade with losartan or the related molecules A-81988 or TCV-116 blocks or attenuates the severe proteinuria observed in rats with reduced renal mass (185, 200), or in SHR with reduced renal mass (201). In the latter study, AT1 DRUGS OF TODAY Vol. 31, No. 7, 1995 473 Hypertension "Pressure dependent" Progressive tissue deterioration (loss of vascular, renal, cardiac, and cerebral function) "Pressure dependent" and "Nonpressure dependent" Increased deaths (strokes, myocardial, infarction, renal failure) "Pressure dependent" and "Nonpressure dependent" Fig. 4. The role of Ang II in cardiovascular disease. blockade with TCV-116 was reported to increase survival (201). In stroke-prone SHR, losartan or TCV-11974 reduced proteinuria and increased survival (202, 203). After uninephrectomy, streptozotocin or puromycin treatment, losartan reduced the proteinuria noted in untreated animals (115, 204-206). The reductions in GFR following unilateral urethral obstruction or ochratoxin were also reversed by losartan at doses of 10-20 mg/kg/day p.o. (207, 208). The elevated blood pressure, proteinuria and glomerulosclerosis characteristic of genetically hyperlipidemic (Imai) rats were blocked by both enalapril and losartan, suggesting that Ang II acting through the AT1 receptor plays a pathological role (209). In a model of passive Heymann nephritis, the decrease in GFR and proteinuria were blocked more by enalapril than losartan, suggesting that enalapril acts through a non-Ang II mechanism (210). Recently, TCV-116 has been shown to inhibit the decrease in GFR following antiglomerular basement membrane antibody-induced nephritis if given 2 hours before the antibody injection, but not if given for 1 week starting from day 3. The benefit appears to be dependent on the state of activation of the RAS (211). From the collective experience to date, Ang II appears to exert significant renal tissue-damaging effects where renal mass or cellular function is compromised. It is not clear how much is "pressure-dependent" and how much is "pressure-independent". It is clear, however, that AT1-selective blockade produces significant renal tissue protection in the majority of experimental models. The relative lack of effect of losartan in passive Heymann nephritis suggests that Ang II may not be involved in this model. In cardiac tissue, Ang II has multiple sites of action, including myocytes, fibroblasts, sympathetic nerves, adipocytes and coronary vascular endothelial and smooth muscle cells (212-214). The Ang II subtype and the distribution of receptors vary with species and functional state (e.g., normal vs. failing heart). The heart appears capable of generating its own Ang II (so-called cardiac tissue RAS) (214) and, in addition, may generate Ang II by non-ACE pathways (e.g., chymase) (215, 216). The relative contribution of locally generated or non-ACE-generated Ang II to the overall actions of Ang II in the normal and/or failing heart is largely unknown. Unlike in the kidney where the functional role of Ang II has been more clearly established, the role of Ang II in the heart is not fully understood. The increase in survival in patients with heart failure treated with inhibitors of Ang II synthesis (SOLVD, CONSENSUS, SAVE trials; for review, see 28), however, has suggested that Ang II has an important pathological role. Ang II induces a number of cellular changes in isolated myocytes involving IP3 concentrations, intracellular calcium release, cAMP levels and proto-oncogene expression (81), and each of these responses to Ang II is blocked by losartan. Cardiac sympathetic nerves release norepinephrine and Ang II enhances this release, presumably through a presynaptic effect. In isolated guinea pig left atria, losartan (1 M), but not PD-123319 (100 M), blocked the Ang ll-enhanced release 474 (217). Likewise, in human right atrial appendages, AT1 blockade with EXP-3174 or nonspecific blockade with saralasin inhibited the enhanced norepinephrine release (90). Interestingly, as described above, untreated SHR display an abnormal response to Ang II, which can be reversed by chronic losartan treatment (218). The inotropic effects of Ang II in vivo are not well understood. In isolated rat papillary muscle, the maximum response is 41-75% of the response to isoproterenol. This response is blocked by losartan, but not by PD-123319 or PD-121981 (219, 220). In isolated human atrial tissue, Ang II has positive inotropic effects, but not in right or left ventricular tissue (221, 222). The response to Ang II was blocked by losartan or saralasin, but not by propranolol or prazosin (222). Fibroblasts are abundant in the failing heart and participate in the cardiac remodeling of the failing heart (223). In cultured neonatal rat heart fibroblasts, Ang II increases DNA and protein synthesis and mitogen-activated kinase (224-226). In a preliminary report using adult rat fibroblasts, both losartan and PD-123177 (AT2) had an inhibitory effect (227). In cultures of human fibroblasts, Ang II increased collagen synthesis ([3H]-proline incorporation), and this response was blocked by PD-123319, but not by ICID8731 (a nonpeptide AT1 antagonist), suggesting an involvement of the AT2 site. In vivo, however, losartan and ACE inhibition (nonseiective) produced comparable reductions in collagen content in rats after coronary ligation (228, 229). A recent report, •• however, shows that cultures of human fibroblasts have a growth response to Ang II, which is not blocked by either losartan or PD-123319, suggesting an alternative, as yet uncharacterized, receptor subtype (230). AT1 receptor antagonism in experimental models of heart failure "Heart failure" (broadly defined as a progressive loss of cardiac function or rise in left ventricular enddiastolic pressure [LVEDP]) can be induced in a variety of animal species by a variety of methods (212, 231). Although none of these models perfectly reflects the human clinical syndrome of heart failure, they can provide insight into the potential pathological role of Ang II and the beneficial effects of Ang II receptor antagonists. The degenerative cardiac changes observed in S40 virus-treated mice are reduced by TCV-116, suggestive of a role of Ang II (232). In genetically myopathic hamsters, AT1 receptor blockade with SR-47436 prevents the progressive loss of ventricular function (233). In socalled "high-output" failure induced by aortocaval shunt, AT1 receptor blockade with losartan reverses MEDICAMENTOS DE ACTUALIDAD the hemodynamic and hypertrophic changes characteristic of this model (234, 235). Similar results were reported for ACE inhibitors and other AT1 receptor antagonists (236-238). Treatment with losartan (10 mg/kg/day twice daily by gavage) and captopril (1 g/l in the drinking water) for 3 weeks beginning 7-8 days after aortocaval shunt surgery improved hemodynamics, decreased water retention and reversed cardiac hypertrophy (239). These data show that Ang II has an important pathological role in this model of heart failure. Cardiac failure produced by rapid ventricular pacing is characterized by marked elevation in LVEDP and reduced cardiac output (CO) in both sheep and dogs (240-242). Losartan given as bolus injections produced marked but short-lived reductions in LVEDP and increases in CO in sheep. The magnitude of the changes was the same as noted with captopril (240). The short duration of action reflects the limited half-life of losartan in sheep. In dogs, losartan, EXP-3174, SR-74736 and TCV-116 have all been shown to have beneficial effects. However, each is a biphenyltetrazole and has a limited duration of action in dogs (241-245). These studies should be repeated with chronic infusions of these agents to assure 24-hour AT1 receptor blockade. Losartan has been studied in rats with coronary artery ligation-induced heart failure. The results have been inconsistent. The first report showed that losartan and captopril given for 2 weeks starting 2 weeks after ligation produced comparable reductions in LVEDP (189). A subsequent study showed that losartan administered s.c. via osmotic pump reduced left ventricular hypertrophy and collagen synthesis, but did not affect DNA synthesis or restore the hemodynamic response to saline infusion (228). The latter group had shown that captopril, but not benzapril, restored the functional response to saline (246). In a study in which losartan or captopril was administered in the drinking water for 2 weeks starting 3 weeks after coronary artery ligation, both agents lowered arterial pressure, but only captopri! reduced renal vascular resistance (247). In a more recent study, moexipril or losartan was given for 1 week before coronary artery ligation and then for an additional 6 weeks. In this study, moexipril lowered LVEDP and reduced infarct size, whereas losartan had no effect on either parameter (248). In a comparative trial of enalapril, losartan and placebo, the treatments were begun 7 days after coronary artery ligation and continued for 6 weeks. In this study, interstitial fibrosis determined from the perfusionfixed heart was elevated in the placebo-treated animals and reduced by both enalapril and losartan. Both methods of RAS inhibition also restored the minimal coronary vascular resistance (229). AT 1 DRUGS OF TODAY Vol. 31, No. 7, 1995 receptor blockade with TCV-116 and delapril has also been reported to decrease left ventricular hypertrophy and LVEDP, Importantly, treatment of rats with coronary artery ligation for up to 1 year with either losartan or captopril has been completed. The preliminary results indicate that the losartan-treated animals have a mean survival time of 246 days versus 149 days for the captopril group (249). This study provides important support for the ongoing trial of losartan in heart failure. Commentary At the time of its 1994 market introduction in Europe, losartan had been studied more extensively than any previous antihypertensive dug. This reflects scientific and clinical interest in the "Ang II system" and the fact that losartan is the first specific nonpeptide Ang II receptor antagonist that is devoid of intrinsic agonist activity. The oral activity and long duration of action of losartan have greatly facilitated chronic studies in rats. Losartan has proven highly useful as a prototype of the AT1 antagonist as a tool to explore Ang II receptor heterogeneity. Although a role for the AT2 site remains elusive, the search for its physiological role continues (250, 251). The use of losartan and other AT1 -selective antagonists to more precisely characterize the Ang ll-dependent effects of ACE inhibitors has only just begun. Theoretically, AT1 receptor antagonists should block Ang II generated by both ACE and non-ACE (alternative pathways) synthesis, and do not affect bradykinin. While decreasing Ang II production, ACE inhibition blocks the availability of Ang II to both receptor: subtypes and potentiates bradykinin. The majority of data generated to date show that AT1 receptor blockers and ACE inhibitors produce equivalent effects both in vitro and in vivo, suggesting that AT1 receptors mediate the principal physiological and pathophysiological effects of Ang II. These findings in preclinical studies have now been confirmed in early human clinical trials. Acutely, losartan (and other AT1 antagonists) antagonize Ang II and lower blood pressure in hypertensive patients. The long-term studies of losartan in hypertensive patients with and without nephropathy and patients with heart failure will provide a further refinement of our understanding of the pathological role of Ang II and the therapeutic value of specific AT1 receptor blockers. Clinical Experience with Losartan and Other AT1-Selective Antagonists The majority of human clinical trials of AT1 -selective antagonists completed to date have been with losartan (Table VI). Clinical trials with a number of other AT1-selective antagonists are now under way 475 (Table VII). The general goal for the phase I clinical trials has been to establish safety and tolerability, oral bioavailability and the blockade of Ang II pressor effects in normal volunteers. From these studies, the range of doses for short-term safety and efficacy trials in hypertensive patients was determined. The results of the dose-finding trials in hypertensive patients (phase II) then provided the dosing parameters for the longer term, multicenter trials (phase III) and studies in special populations (e.g., severe hypertension, renally impaired, elderly) or trials versus other comparative compounds. In addition, studies with concomitant administration of hydrochlorothiazide were also performed (252-254). Losartan has been studied in 16 double-blind, placebo-controlled trials. At the time of regulatory submission of the New Drug Application (NDA) with the Federal Drug Administration (FDA) (December, 1993), data from more than 3300 subjects were included. Currently, more than 1700 patients have received losartan for more than 1 year. Efficacy and safety data have been collected from over 4058 patients/subjects enrolled in clinical trials (302). Clinical trials in normal subjects Losartan is an orally active Ang II antagonist in man. The blockade of Ang ll-induced pressor effects in man was demonstrated in two studies performed in normal subjects in whom the blood pressure response to i.v. infusion of Ang I or Ang II repeated several times over 24 hours was monitored by photoplethysmography (Finapres; Ohmeda, Englewood, CO, USA). In the initial study, single oral doses of losartan (2.5, 5,10, 20 and 40 mg) were studied versus placebo. Doses of 10, 20 and 40 mg of losartan produced dose-related inhibition of the systolic blood pressure response to Ang I (converted to Ang II). Demonstrable inhibition of Ang II was evident at 24 hours with the 40-mg dose. At these doses, plasma levels of immunoactive Ang II rose, whereas the fall in plasma aldosterone was not different from placebo (112, 255). In the second study, losartan (20 and 40 mg) was given orally to volunteers once a day for 8 days. The i.v. Ang II challenge was given several times on days 2, 4 and 8. Losartan produced a dose-related inhibition of the systolic and diastolic blood pressure response to Ang II. Similar maximum inhibition of Ang II was achieved on days 2, 4 and 8, suggesting that acute tolerance to the receptor antagonism did not develop. The 20- and 40-mg doses of losartan significantly elevated PRA and Ang II levels on days 1 and 8 (268). Pharmacokinetic studies were also carried out in normal volunteers. Plasma was analyzed for losartan and the active metabolite EXP-3174. Because of MEDICAMENTOS DE ACTUALIDAD 476 Table VI: Summary of clinical experience. Patient description Clinical observation Normotensive volunteers Ang II antagonism Pharmacokinetics PRA and Ang II levels Renal function Forearm blood flow 255 256, 257 258 105,259 260 Essential hypertensives PRA, Ang II, aldosterone Renal hemodynamics Ambulatory blood pressure 261 262, 263 264 Antihypertensive effect Heart failure patients References 263, 265-271 Chronic renal insufficiency Effect on proteinuria Insulin sensitivity Cough Circadian periodicity of BP Safety EXP-3174 i.v. hemodynamics 272 273 274 275, 276 277 83 278 Acute hemodynamic effects 12-Week hemodynamic effects Exercise performance 279, 280 281 282 Ang II - angiotensin II; PRA = plasma renin activity; BP = blood pressure. Table VII: Selective AT1 receptor antagonists in clinical trials. Compound Other designation Status Losartan DuP-753, MK-954 Marketed in U.S., some countries in Europe References Candesartan TCV-116 Phase III 283-285 Valsartan CGP-48933 Phase III 286 (see Table VI) Irbesartan SR-47436/BMS-18295 Phase III 287, 294 Telmisartan BIBR-0277 Phase II 295, 296 Eprosartan SK&F-108566 Phase II 297 SC-52458 Phase I 298, 299 TAK-536 Phase I 300 Other drugs in clinical trials without published data include E-4177, HN-65021, GR-117289 (zolasartan), UP-2696, LRB/081, MK-996 (L-158,282) and ANA-756. its slow clearance, EXP-3174 gave high plasma levels based on the AUC and had a longer terminal half-life (t1/2). Because blood levels of EXP-3174 paralleled the antagonism of Ang II, the authors concluded that the long-lasting Ang Il blockade following losartan is largely due to the active metabolite EXP-3174 (256) (Fig. 5). Unlike ACE inhibitors, losartan does not potentiate the vasodilator response to bradykinin in the human forearm (260). On different occasions, eight normal subjects received single doses of losartan 20 mg, losartan 100 mg, enalapril 10 mg and placebo in a double-blind, four-period crossover study (260). Forearm blood flow was measured by venous occlusion plethysmography during infusions of Ang I, Ang II and bradykinin. At 4-6 hours after dosing, losartan inhibited the vasoconstrictor responses to Ang I and Ang II in a dose-related manner, but it did not potentiate the vasodilator response to bradykinin. Enalapril, in contrast, selectively blocked the vasoconstrictor response to Ang I (without changing Ang II) and significantly augmented the vasodilator response to DRUGS OF TODAY Vol. 31, No. 7, 1995 477 Fig. 5. Top: Time profile of the mean plasma concentrations of the parent compound (closed symbols) and its active metabolite EXP-3174 (open symbols) after single oral administration of losartan (squares, 40 mg; diamonds, 80 mg; triangles, 120 mg) in six volunteers. For purposes of clarity, SD values are reported for the highest oral dose and for the active metabolite only. Bottom: Time profile of the mean response to exogenous angiotensin II challenge in six volunteers receiving placebo (no symbols) and three doses of losartan (asterisks, 40 mg; diamonds, 80 mg; triangles, 120 mg). The ordinate is expressed as a percentage of the baseline predose response. For purpose of clarity, SEM are reported for placebo and 120 mg only. (Reproduced with permission from Munafo et al. Drug concentration response relationships in normal volunteers after oral administration of losartan, an angiotensin II receptor antagonist. Clin Pharmacol Ther 1992, 51:513-521.) bradykinin. These data are consistent with the lack of effect of losartan on ACE activity and on the smooth muscle effects of bradykinin (154, 302). Losartan increases PRA and Ang II levels in normal subjects (258), presumably through inhibition of the negative feedback mechanism in the kidney. Losartan 100 mg/day was administered for 8 days. PRA (expressed as ng of Ang I generated/ml/h) was measured by radioimmunoassays and Ang II levels were measured by high-performance liquid chromatography (HPLC) plus a radioimmunoassay during the run-in period and 6 hours after dosing. The PRA increased from 1.2 ± 0,6 (run-in) to 12.0 ± 6.3 (after the first dose) and to 9.6 ± 4.9 (after the last dose). The Ang II levels increased from 4.3 ± 1.7 ng/ml (run-in) to 77.4 ± 33 ng/ml (after the first dose) and to 45.7 ±14.1 ng/ml (after the last dose). Thus, the incremental increase in Ang II was somewhat less after the last dose than after the first dose (p < 0.05). Similar changes in the RAS hormones have been observed in hypertensive patients (261). In these studies, losartan 25 and 100 mg/day 478 MEDICAMENTOS DE ACTUALIDAD Fig. 6. Graphs show geometric mean plasma angiotensin II measurements at placebo run-in visit and geometric mean fold changes of angiotensin II during treatment. At 0 hours, changes in the 100-mg losartan group were significantly different from run-in at weeks 2 and 6 and significantly different from the placebo group at week 2. In the 20-mg enalapril group, changes were significantly different from run-in and the placebo group at week 6. (Reproduced with permission from Hypertension, Copyright 1995, American Heart Association, Ref. 261.) increased PRA and Ang II levels at 4 hours, and the increase in both parameters was less pronounced at week 6 than at week 2 (Fig. 6). The absorption, distribution, metabolism and excretion characteristics of losartan include: .1) good bioavailability, e.g., 30%, 2) conversion to EXP-3174 via cytochrome P-450 in the liver, and 3) elimination of losartan and EXP-3174 via both the hepatic and renal routes. Losartan is highly protein-bound (98.6-98.8%) (1.4 ± 0.2 to 1.2 ± 0.1% unbound at concentrations of 0.5-5.0 g/ml). EXP-3174 is more than 99.7% bound (0.2 ± 0.0% unbound at concentrations of 0.1-10 g/ml). Binding to -acid glycoprotein is negligible. In vitro concentrations of warfarin up to 20 g/ml, diazepam up to 35 g/ml, naproxen up to 50 g/ml or ibuprofen up to 20 g/ml did not affect losartan binding. Supratherapeutic concentrations of acetylsalicylic acid (ASA; 300 or 3000 g/ml), naproxen (500 g/ml) or ibuprofen (200 g/ml) did significantly increase the percent of free losartan (303). In formal interaction studies in man, the blood levels of losartan were not affected by phe-nobarbital, cimetidine or ketoconazole. Losartan and EXP-3174 have a volume of distribution of 34 and 12 liters, respectively (301). Losartan is converted to the free carboxylic acid EXP-3174, which is a potent AT1 receptor antagonist (see above), in rats and man (256). In these two species, losartan undergoes extensive first-pass metabolism by hepatic P-450 enzymes (110). In human liver slices, the major metabolite is EXP-3174. In dogs, the glucuronide is the primary product, with little EXP-3174 formed (109). The extent of conversion is approximately 14% in man, 20% in rats and < 1% in dogs. In these studies, a sensitive (5 ng/ml for plasma and 10-20 ng/ml for urine) HPLC assay for the simultaneous detection of both compounds was described (304). Losartan is excreted in man via both the bile and the urine, and following oral [14C]-losartan, approximately 35% of the radioactivity is recovered in the urine and about 60% in the feces. The plasma levels of losartan (AUCs) in patients with a creatinine clearance of 30 ml/min or less were increased by 50% and were 2-fold greater in patients on hemodialysis; however, no dose adjustment is recommended (301) unless volume depletion is present. In patients with mild to moderate hepatic impairment, the blood levels (AUCs) of losartan and the metabolite EXP-3174 have been shown to be elevated 5- and 1.7-fold, respectively, and reduction of the starting dose has been recommended (301). Clinical trials in salt-depleted volunteers Losartan has been shown to lower blood pressure in sodium-depleted normal subjects (105,259). Subjects were studied following a single dose of losartan on four occasions 2 weeks apart. Each was pretreated with furosemide (40 mg twice daily) for 3 days prior to the study day, and then given placebo salt replacement ("salt-depleted") or active salt replacement ("salt-replete") (105). During salt depletion, a single dose of losartan 100 mg significantly lowered both supine (-24 ± 9 mmHg) and erect (-33 ± 1 5 mmHg) blood pressure. In contrast, when the subjects were salt-repleted, losartan had no significant effect on blood pressure compared to placebo. Creatinine clearance was transiently reduced by losartan in salt-depleted subjects (47 ± 54 ml/min for losartan, 161 ±37 ml/min for placebo), but not in salt-replete subjects. Activation of the RAS by the low-salt diet was confirmed with the demonstration DRUGS OF TODAY Vol. 31, No. 7, 1995 479 that PRA was significantly elevated by salt depletion. It was concluded that care should be taken in treating vigorously salt-depleted patients (105). In the second normal volunteer study, losartan or placebo was administered to 23 healthy subjects on a high-sodium (200 mmol/day) or low-sodium (50 mmol/day) diet in a randomized, double-blind, crossover design (259). To maintain adequate urinary flow rates, the patients were hydrated throughout the study day. When the subjects were on a low-sodium diet, losartan significantly increased urinary sodium excretion (from 115 ± 9 to 207 ± 21 mmol/min) and potassium excretion (from 3.5 ± 0.2 mmol/min to 11.1 ± 0.5 mmol/min). During the high-sodium diet, losartan produced somewhat smaller effects, which did not reach statistical significance. Uric acid excretion increased to 9.66 ± 0.6 mmol/min from a baseline of 3.1 ± 0.2 mmol/min. In these water-loaded patients, losartan did not acutely affect blood pressure, GFR or renal blood flow under either dietaiy sodium condition. Uricosuric effect One surprising finding during the clinical development programs was the observation of a transient uricosuria following treatment with losartan. Losartan has been shown to increase the urinary excretion of uric acid in normal subjects (256) and hypertensive patients (269). The uricosuric effect of losartan does not appear to be related to AT1 receptor blockade or renin status. The early time course of the transient increase in uric acid excretion suggests that it is an effect of losartan rather than the metabolite (259). This is supported by the apparent lack of effect on uric acid excretion of EXP-3174 when administered i.v. (278). Analysis of the overall experience with losartan in controlled clinical trials showed that plasma uric acid decreased by approximately 0.4 mg/dl (301). The mechanism of this uricosuric effect is unknown. Losartan does not appear to inhibit xanthine oxidase or to stimulate intestinal water transport (305). Antihypertensive effects The results of several large multicenter trials with losartan in hypertensive patients have been published (263, 270) (see Table VI). The clinical database for losartan includes the results of 16 doubleblind controlled trials totaling 2805 losartan-treated patients (302). The efficacy and safety of once-daily losartan (10,25,50,100 or 150 mg) have been demonstrated in an 8-week, double-blind trial in patients with mila to moderate essential hypertension (Fig. 7) (263). Five hundred and seventy-six patients were randomly allocated to receive different doses of Fig. 7. Graphs show changes in diastolic and systolic pressure by weeks in patients with mild to moderate essential hypertension. Shown is the mean change (mmHg) in trough (24 hours after dosing) supine blood pressure by week. Top: changes in diastolic pressure; Bottom: changes in systolic pressure. LOS = oral losartan potassium (doses are in mg once daily); ENAL 20 = enalapril maleate (∆ , 20 mg given once daily);● , placebo; ▲, LOS, 10;■ , LOS 25; ♦, LOS 50; ▼ , LOS 100; O, LOS 150). (Reproduced with permission from Hypertension, Copyright 1995, American Heart Association, Ref. 263.) losartan, placebo or the positive control enalapril (20 mg). After 8 weeks of treatment, mean reductions from baseline in supine systolic/diastolic pressure 24 hours after dosing (trough) for losartan 10,25, 50, 100 and 150 mg were 7.6/7.9, 7.8/6.8, 13.0/10.1, 8.9/9.9 and 10,5/9.7 mmHg, respectively. The values for enalapril and placebo were 14.7/11.2 and 3.8/5.6 mmHg, respectively. Losartan doses of 10 and 25 mg did not result in clinically significant differences from placebo (263). As shown in Figure 7, the blood pressure changes are essentially identical to those observed with enalapril at a dose of 20 mg. The placebo-corrected trough-to-peak ratios of the mean changes in supine diastolic blood pressure after 8 weeks were 78% (10 mg), 23% (25 mg), 60% (50 mg), 72% (100 mg) and 49% (150 mg) for losar- 480 tan, indicating a sustained 24-hour effect, which is not a result of excessive effects at peak (4-6 hours). Losartan was generally very well tolerated and no dose-related trends in adverse experiences were noted. Headache was the most common adverse effect in most treatment groups, including the placebo group (263). Antihypertensive effects of EXP-3174 In patients with essential hypertension, the acute hemodynamic and renal effects of EXP-3174 administered i.v. appear to be consistent with AT1 receptor blockade. EXP-3174 (20 mg infused over 4 hours) significantly reduced diastolic pressure compared to placebo beginning approximately 100 minutes after the initiation of the infusion (278). The maximum blood pressure reduction did not occur until 8 hours after initiation of the infusion, even though the maximum blood levels (324 ng/ml) were achieved within the first 20 minutes of infusion. The reason for this delayed onset is not presently known, but may reflect the low volume of distribution (12 l vs. 34 I for losartan) and the delay in equilibrating with different tissue compartments. Only modest changes (not-significant) in PRA were observed. Urinary excretion of uric acid, Na+, K+ or chloride was not significantly altered. Antiproteinuric effects in nondiabetic hypertensives Losartan demonstrated beneficial effects on urinary protein excretion in nondiabetic hypertensive patients (273) with creatinine clearance of > 60 ml/ min and protein excretion exceeding 2 g in 24 hours. In an open-label, crossover study, losartan (50 and 100 mg) and enalapril (10 and 20 mg) (Fig. 8) produced comparable reductions in protein excretion, reduction in blood pressure (15.1% vs. 17.3%), increase in effective renal plasma flow (ERPF; 13.3% vs 13.1%) and decrease in filtration fraction (15.1% vs. 14.6%). These findings have been extended to an 11-month, single-blind study comparing losartan 50 or 100 mg/day to placebo (306). In this study, urinary excretion of total protein, albumin and immunoglobulin G (IgG) was decreased in a dose-dependent manner. At the high dose, serum uric acid fell from 0.43 ± 0.02 mmol/l to 0.39 ± 0.02 mmol/l, and serum potassium decreased from 4.7 ±0.1 mmol/l to 4.6 ± 0.1 mmol/l. Concomitant administration with hydrochlorothiazide Diuretics are known to produce additive blood pressure effects when combined with other antihypertensive drugs, particularly those that inhibit the RAS. A fixed-dose combination of losartan (50 mg) MEDICAMENTOS DE ACTUALIDAD and hydrochlorothiazide (12.5 mg) has been_ approved for the treatment of hypertension in the United States. This was based on the results of three double-blind, placebo-controlled trials. In one study, hydrochlorothiazide (6.25,12.5 or 25 mg) was given to patients who did not achieve a target diastolic pressure of 93 mmHg after 4 weeks of losartan 50 mg alone. Statistically significant additional reductions in diastolic and systolic pressures (compared to placebo) were achieved only with 12.5 or 25 mg hydrochlorothiazide, e.g., systolic/diastolic: -12/-9 mmHg and -16/-11 mmHg, respectively (253). Similar findings were reported in another study in which losartan 25, 50 or 100 mg was given to patients who did not achieve target blood pressure on hydrochlorothiazide 25 mg (270). The concomitant administration of losartan 50 mg and hydrochlorothiazide 12.5 mg has been evaluated by Arcuri et al. (254). By extrapolation to other studies, this study demonstrated an approximately 50% increase in efficacy over losartan monotherapy. The increases in serum glucose, uric acid and potassium induced by hydrochlorothiazide appeared to be reduced in the losartan-treated patients compared to placebo (Fig. 9) (264). Safety and tolerability Losartan is very well tolerated in patients with essential hypertension. Approximately 2800 patients/subjects have been treated with losartan alone or in combination with other antihypertensive agents in double-blind clinical trials. On the basis of data pooled from these trials, it was concluded that losartan has an excellent tolerability profile comparable to placebo (83). Table VIII summarizes clinical adverse experiences. The most frequent clinical adverse effects considered by the investigator to be drug-related were headache (4.2%), dizziness (2.4%) and asthenia/fatigue (2.0%). Only dizziness occurred more often in losartan-treated than in placebo-treated patients (2.4% vs. 1.3%). Withdrawals due to adverse events occurred in 2.3% of patients receiving losartan alone and in 2.8% of those receiving losartan plus hydrochlorothiazide. Withdrawals occurred in 3.7% of the placebo-treated patients and in 2.5% of the ACE inhibitor-treated patients. In contrast, the incidence of withdrawals was 8.8% for blockers and 9.3% for calcium channel blockers. In patients with a history of "ACE cough" who were subsequently challenged with lisinopril, losartan was associated with significantly less cough than lisinopril (276). In this trial, hypertensive patients with a history of ACE inhibitor-associated cough were first challenged with lisinopril to confirm the presence of cough, then dechallenged with placebo during the washout period, during which time the cough had to DRUGS OF TODAY Vol. 31, No. 7, 1995 481 Fig. 8. The effects (median with 95% Cl) of angiotensin II receptor antagonism (50 and 100 mg once daily) and ACE inhibition (10 and 20 mg once daily) in 11 patients with proteinuria due to no nondiabetic renal disease. Shaded areas represent study periods in which active treatment (Ang II, angiotensin II antagonist; ACE inhibition) was given, while nonshaded areas represent study periods in which placebo was given. Changes in blood pressure ( ■ ) and urinary protein excretion (●) are depicted in the upper panel, changes in glomerular rate (∆ ), effective renal plasma flow (□ ) and filtration fraction (O) are depicted in the lower panel. Parameters are expressed as percentage change from baseline. *p < 0.05 vs. baseline (two-way ANOVA, Friedman). (Reproduced with permission from Gansevoort et al. Is the antiproteinuric effect of ACE inhibition mediated by interference in the renin angiotensin system? Kidney Int 1994, 45(3): 861-867. Ref. 273.) disappear. Patients were then randomized in a double-blind fashion to lisinopril, hydrochlorothiazide (a negative control) or losartan. The incidence, severity and frequency of cough were assessed by a self-administered questionnaire and visual analog scale (275). The results indicated that the incidence of cough with losartan (29.2%) was significantly less than with lisinopril (71.7%) and was similar to that with hydrochlorothiazide (34.1%). These results confirmed differences between ACE inhibition and losartan in the large phase III trial database for spontaneous reports of cough (ACE inhibitors 8.8% vs. losartan 3.1%) (Table VIII). Clinical trials in patients with heart failure Losartan is not approved for the treatment of heart failure, but it is currently under evaluation in such patients. Acute and multiple-dose hemodynamic studies and two pilot exercise studies have been completed (280, 281). Two multicenter exercise efficacy trials and an efficacy and tolerability study in elderly patients with heart failure versus captopril (ELITE Trial, Evaluation of Losartan in the Elderly) are ongoing (see below). Losartan produced beneficial acute hemodynamic effects in patients with heart failure consistent with Ang II system blockade (280). The acute hemodynamic and neurohumoral response to single doses of losartan (5,10,25,75 or 150 mg) or placebo was studied in 66 patients with New York Heart Association functional class II, III or IV heart failure and an ejection fraction of < 40% (280). Hemodynamic and neurohumoral measurements were obtained at selected times for 24 hours post-treat- 482 Fig. 9. Mean serum concentrations of potassium, glucose and uric acid at baseline, after 4 weeks of monotherapy with placebo or various dosages of losartan, and after an additional 2 weeks during which hydrochlorothiazide, 12.5 mg/day, was given in combination with the previous treatment. (Reproduced with permission from Arch Intern Med 1995, 155(4): 405-411, Copyright 1995, American Medical Association, Ref. 264.) ment. Losartan produced a dose-related fall in mean arterial blood pressure and capillary wedge pressure at doses up to 25 mg. The 75- or 150mg doses of losartan had no additional effect. Modest increases in cardiac index were noted at all doses. Heart rate was not changed by any of the doses of losartan evaluated. Plasma renin activity and Ang II levels increased in a doserelated fashion up to 150-mg. Maximum values were observed at 4-6 hours post-treatment and remained elevated for 24 hours. Modest reductions in plasma norepinephrine and aldosterone were noted. The acute and chronic hemodynamic responses to 12-week treatment with losartan (2.5,10, 25 or 50 mg once daily) were assessed in 134 patients with MEDICAMENTOS DE ACTUALIDAD symptomatic heart failure and impaired left ventricular function (ejection fraction < 40%) (281). Invasive 24-hour hemodynamic assessment was performed after the first dose and after 12 weeks of treatment. Acutely, systemic vascular resistance (SVR) and mean arterial pressure were significantly reduced following the 50-mg dose. The 25-and 50-mg doses of losartan increased PRA and Ang II levels consistent with the previously reported study (280). After 12 weeks, similar effects were seen on SVR and blood pressure (maximum decrease in SVR compared to placebo at 5 hours with 50 mg). In addition, pulmonary capillary wedge pressure fell with 2.5,25 and 50 mg, cardiac index rose with 25 and 50 mg, and heart rate was lower in all treatment groups. Losartan was well tolerated, with persistent beneficial hemodynamic effects at 12 weeks, the greatest effect being seen with 50 mg. The published experience with losartan in heart failure is limited at this time. In general, it appears that losartan has beneficial hemodynamic effects in patients with symptomatic heart failure. The decrease in pulmonary capillary wedge pressure after 12 weeks of treatment and the lack of effect of losartan on heart rate are consistent with prior experience with ACE inhibitors. In addition, a higher incidence of progression of heart failure was observed in the placebo and low-dose groups compared to the higher dose groups, suggesting that losartan may exert a beneficial effect in heart failure. At the present time, there are no published data on other AT1-selective antagonists in patients with heart failure. Clinical trials with losartan in heart failure are continuing with exercise studies and a safety study in elderly patients (the ELITE trial). Results of a 12-week pilot trial with losartan at 25 and 50 mg versus enalapril at 10 mg b.i.d. suggested that losartan is well tolerated in patients with heart failure, and exercise capacity and clinical status were similar after treatment with losartan or enalapril (307). Conclusions Losartan is the first of a new class of therapeutic agents. A large number of related molecules are at present in various stages of development (57, 58). Although many of these compounds are more potent than losartan (lower doses producing maximum inhibition of Ang II), full blockade of AT1 receptors is achieved by all losartan-related molecules. Comparative trials with various AT 1 antagonists are not yet available, but it is anticipated that all agents will produce approximately equal antihypertensive efficacy. As with the development of the ACE inhibitor class of agents, it is likely, however, that studies will be available in the near future that will attempt to DRUGS OF TODAY Vol. 31, No. 7, 1995 483 Table VIII: Percentage of patients reporting clinical adverse experiences. Losartan (n = 2085) Losartan + HCTZ (n = 858) Placebo (n = 535) ACE inhibitors (n = 239) 46.8 45.0 52.0 50.6 57.4 53.5 48.3 Percentage with any 15.3 drug-related adverse experience 14.8 15.5 24.7 26.5 23.3 18.1 Percentage with any adverse experience -Blocker (n = 68) Calcium channel blocker (n = 43) HCTZ (n = 271) Asthenia/fatigue 3.8 2.9 3.9 6.7 5.9 0.0 5.5 Cough 3.1 2.6 2.6 8.8 0.0 2.3 4.1 Diarrhea 1.9 1.5 1.9 2.9 0.0 4.7 Dizziness 4.1 5.7 2.4 6.3 7.4 2.3 Edema 1.7 1.3 1.9 1.7 1.5 14.0 19.1 14.0 14.1 7.7 17.2 10.9 Upper respiratory infection Headache 6.5 6.1 5.6 5.4 Insomnia 1.1 0.5 0.7 0.8 1.5 4.4 7.0 2.3 0.4 4.1 1.8 14.0 5.5 1.1 ACE - angiotensin-converting enzyme; HCTZ = hydrochlorothiazide. *During treatment or within 14 days after discontinuing the test drug. (Adapted with permission from Amer J Cardiol, Ref. 83.) differentiate newer agents on the basis of bioavailability, onset of action, duration of action, tissue penetration, or whether an active metabolite is required for full biological activity. From a practical point of view, losartan is a once-a-day drug that blocks all of the cardiovascular effects of Ang II whether formed by the classical RAS pathway or through alternative biosynthetic pathways. The specific site of action of losartan at the AT1 receptor appears to provide both the desired blockade of the Ang II system and an excellent tolerability profile. The hypothetical concern about elevated circulating Ang II during losartan treatment interacting with AT2 sites does not appear to be justified based on current clinical experience (301). Although the role of the AT2 sites remains largely unknown, therapeutic doses (50 or 100 mg/day) for periods of up to 2 years in patients with hypertension have continued to show good tolerance (83). Losartan lowers blood pressure comparable to other classes of currently used antihypertensive agents, including ACE inhibitors, calcium channel blockers, -blockers and diuretics (308). Fewer adverse reactions have been noted with losartan than with felodipine, hydrochlorothiazide or atenolol. Although ACE inhibitors were generally well tolerated in comparative trials with losartan, the incidence of dry cough was significantly greater (8.8% for ACE inhibitors, and 2.6 and 3.1 % for placebo and losartan, respectively) (83). The long-term benefits of losartan in patients with hypertension are unknown. Although more than 1700 patients have taken losartan for more than 1 year in extensions of clinical trials and for which adverse experiences have been recorded, no morbidity/mortality data are currently available. A wealth of preclinical data supports the probable efficacy of the chronic use of losartan in hypertensive patients and in heart failure. A 5-year endpoint (cardiovascular morbidity and mortality) trial is, however, under way in Scandinavia and the United States, This trial, designated the LIFE Trial (Losartan intervention for Endpoint Reduction), is a double-blind, comparative trial of losartan- and atenolol-based regimens in hypertensive patients with left ventricular hypertrophy identified by sensitive ECG criteria. This trial will include approximately 8300 patients and is scheduled to be completed by the end of the year 2000. Beneficial effects of fosartan on renal function and proteinuria have been demonstrated in an openlabel, short-term study in patients with proteinuria (273). In addition, a number of preclinical studies have shown that losartan and other AT1-selective antagonists have beneficial effects in various models pf renal dysfunction (see above). Extrapolating the results from preclinical studies, it is anticipated 484 MEDICAMENTOS DE ACTUALIDAD (48-week) trial of losartan treatment in elderly patients with congestive heart failure (CHF) is under way. In this trial, designated ELITE, the effect of losartan on renal safety parameters in elderly patients will be compared to captopril. Losartan is the first of a rapidly expanding class of antihypertensive agents. For the first time, it is possible to selectively inhibit the "angiotensin II system". The results of the long-term studies with losartan and with the other AT1-selective antagonists to follow should clearly define the pathological role of Ang II. From what we know from the early clinical trials with losartan and from the wealth of preclinical data, Ang II acting through the AT1 receptor: 1) behaves as a potent vasoconstrictor, increasing pressure and decreasing blood flow, 2) effects tissue damage at the cellular level in blood vessels, kidney, heart, and possibly brain, and 3) may result in a detrimental cascade of effects resulting in decreased survival. AT1 receptor blockade holds the possibility of preventing or attenuating each of these effects. Fig. 10. Losartan in heart failure. Plots of mean change in pulmonary capillary wedge pressure (mmHg) from pretreatment baseline levels (A) after short-term therapy and (B) after 12 weeks of therapy (placebo, ; losartan 2.5, O; losartan 10 mg, ; losartan 25 mg, Δ ; losartan 50 mg, ◊). Shaded symbols indicate a significant difference (p 0.05) between losartan and placebo groups. (Reproduced with permission from Circulation, Copyright 1995, American Heart Association, Ref. 281.) that losartan will have long-term beneficial effects in clinical syndromes of renal dysfunction, e.g., diabetic nephropathy and nephropathies of other etiologies, but this will require extensive clinical trials. The long-term effects of losartan in heart failure have not been established. Preclinically, losartan has been shown to have beneficial effects on hemodynamics, ventricular remodeling and survival in rodent models of heart failure. In clinical trials in patients with heart failure, losartan produces beneficial hemodynamic effects both acutely (309) and after 12 weeks of treatment (281). A long-term References 1. Timmermans, P.B.M.W.M., Carini, D.J., Chiu, AT., Duncia, J.V., Price, W.A., Wells, G.J., Wong, PC., Wexler, R.R., Johnson, A.L. Angio tensin II receptor antagonists: From discovery to antihypertensive drugs. Hypertension 1991, 18(Suppl. Ill, No. 5): III-136-42. 2. Duncia, J.V., Carini, D.J., Chiu, AT., Johnson, A.L., Price, W.A., Wong, PC., Wexler, R.R., Tim mermans, P.B.M.W.M. The discovery of DuP 753, a potent, orally active nonpeptide angioten sin II receptor antagonist. Med Res Rev 1992, 12(2): 149-91. 3. Carini, D.J., Duncia, J.V. The discovery and development of the nonpeptide angiotensin II receptor antagonists. Adv Med Chem 199.3, 2: 153-95. 4. Smith, R.D., Chiu, AT., Wong, P.C., Herblin, W.F., Timmermans, P.B.M.W.M. Pharmacology of nonpeptide angiotensin II receptor antago nists. Annu Rev Pharmacol Toxicol 1992, 32: 135-65. 5. Timmermans, P.B.M.W.M., Wong, P.C., Chiu, AT, Herblin, W.F., Benfield, P., Carini, D.J., Lee, R.J., Wexler, R.R., Saye, J.M., Smith, R.D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 1993, 45(2): 205-51. 6. Peach, M.J., Dostal, D.E. The angiotensin II receptor and the actions of angiotensin II. J Cardiovasc Pharmacol 1990, 16(Suppl. 4): S25-30. 7. Ferrario, C.M. Importance of the renin-angiotensin-aldosterone system (RAS) in the physiology DRUGS OF TODAY Vol. 31, No. 7, 1995 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. and pathology of hypertension. Drugs 1990, 39(Suppl. 2): 1-8. Alderman, M.H., Madhovan, S., Qoi, W.L., Cohen, H., Sealey, J.E., Laragh, J.H. Associa tion of the renin-sodium profile with the risk of myocardial infarction in patients with hyperten sion. New Engl J Med 1991, 324: 1098-104. Goldblatt, H., Lynch, J., Hanzel, R.E., Summerville, W.W. Studies on experimental hyperten sion: II. The production of persistent elevation of systolic blood pressure by means of renal ische mia. J Exp Med 1934, 59: 347-79. Bright, R. Tabular view of the morbid appear ances in 100 cases connected with albuminous urine with observations. Guy's Hospital Rep 1836,1:380-400. Tigerstedt, R., Bergman, P.G. Kidney and Circ Scand Arch Physiol 1898, 7-8: 223-71. Page, I.H., Helmer, O.M. A crystalline pressor substance (angiotonin) resulting from the reac tion between renin and renin activator. J Exp Med 1940, 71:29-42. Braun-Mendez, E., Fasciolo, E., Leloir, J.C., Munoz, J.M. The substance causing renal hypertension. J Physiol (London) 1940, 98:283-98, Peart, W.S. The isolation of a hypertensin, BiochemJ 1956, 62:520-7. Skeggs, L.T. Jr.,Kahn, J.R., Shumway, N.P. The purification of hypertensin II. J Exp Med 1956,103:301-7. Bumpus, F.M., Schwartz, H., Page, I.H. Synthe sis and pharmacology of the octapeptide angiotensin. Science 1957, 125: 886-7. Schwyzer, R., Iselin, B., Kappelei, H., Riniker, B. Uber die partielle hydrolyse von hypertensinasp- -amiden zu den entsprechenden dicarbonsauren - Hypertensin ll-analoge. Chimica 1957,11:335-6. Khosla, M.C., Smeby, R.R., Bumpus, F.M. Structure-activity relationship in angiotensin II analogs. In: Handbook of Experimental Phar macology, Vol. XXXVII, Angiotensin. Page, I.H., Bumpus, F.M. (Eds.). Springer-Verlag, Berlin 1974,126-61. Khosla, M.C., Bachynsky, E., Forgac, S., Lakios-Cherpas, C., Bumpus, F.M. Angiotensin analogues that selectively augment the force of contraction of the isolated heart. Hypertension 1988, ll(Suppl. I): 138-41. Pals, D.T., Mascucci, F.D., Denning, G.S., Sipos, F., Fessler, D.C. Role of the pressor action of angiotensin II in experimental hyper tension. Circ Res 1971, 29: 673-81. Anderson, G.H. Jr., Dalakos, T.G., Elias, A., Tomycz, N., Streeten, D.H.P. Diuretic therapy 485 and response of essential hypertension to saralasin. Ann Intern Med 1977, 87: 183-7. 22. Anderson, G.H. Jr., Streeten, D.H.P, Dalakas, T.G. Pressor responses to 1-Sar-8-Ala-angiotensin II (saralasin) in hypertensive: subjects. Circ Res 1977, 40(3): 243-50. 23. Buhler, F.R., Laragh, J.H., Baer, L., Vaughan, E.D. Jr., Brunner, H.R. Propranolol inhibition of renin secretion. A specific approach to diagno sis and treatment of renin dependent hyperten sive diseases. New Engl J Med 1972, 287: 1209-14. 24. McDevitt, D.G. Pharmacological characteristics of (3 blockers and their role in clinical practice. J Cardiovasc Pharmacol 1986, 8(Suppl. 6): S5-11. 25. McDevitt, D.G. Position Paper: Different fea tures of beta adrenoreceptor blocking drugs for therapy. In: Frontiers in Hypertension Research. Laragh, J.H., Buhler, F.R., Seldin, D.W. (Eds.). Springer-Verlag, New York 1981, 473-81. 26. Buhler, F.R. Position Paper:Antihypertensive actions of beta-blockers. In: Frontiers in Hyper tension Research. Laragh, J.H., Buhler, F.R., Seldin, D.W. (Eds.). Springer-Verlag, New York 1981,423-35. 27. Leonetti, G., Cuspidi, C. Choosing the right ACE inhibitor - A guide to selection. Drugs 1995,49(4): 516-35. 28. Young, J.B. Reduction of ischemic events with angiotensin converting enzyme inhibitors: Les sons and controversy emerging from recent clinical trials. Cardiovasc Drug Ther 1995, 9(1): 89-102. 29. Burris, J.F. The expanding role of angiotensin converting enzyme inhibitors in the manage ment of hypertension. J Clin Pharmacol 1995, 35(4): 337-42. 30. Skeggs, L.T. Jr. Historical overview of the reninangiotensin system. In: Hypertension and the Angiotensin System: Therapeutic Approaches. Doyle, A.E., Beam, A.G. (Eds.). Raven Press, New York 1984, 31-45. 31. Siegl, P.K.S., Kivlighn, S.D., Broten, T.P., Schaffer, L.W. Pharmacology of angiotensin II recep tor antagonists: Comparison with renin inhibi tors and angiotensin-converting enzyme inhibitors. Unpublished 1994. 32. Kleinert, H.D., Stein, H.H. Specific renin inhibi tors: Concepts and prospects. In: Hypertension: Pathophysiology, Diagnosis, and Management, 2nd Ed. Laragh, J.H., Brenner, B.M. (Eds.). Raven Press, New York 1995, 3065-77. 33. Lunney, E.A., Humblet, C. Renin inhibitor design though molecular modeling. In: Medicinal Chemistry of tha Renin Angiotensin System, 486 21st Ed. Timmermans, P.B.M.W.M., Wexier, R.R. (Eds.). Elsevier, Lausanne 1994, 73-101. 34. Unger, T., Gohike, P. Converting enzyme inhibi tors in cardiovascular therapy: Current status and future potential. Cardiovasc Res 1994, 28(2): 146-58. 35. Struthers, A.D. The clinical pharmacology, of angiotensin converting enzyme inhibitors in chronic heart failure. Pharmacol Ther 1992, 53: 187-97. 36. Keilani, T., Schlueter, W., Batlle, D. Selected aspects of ACE inhibitor therapy for patients with renal disease: Impact on proteinuria, lipids and potassium. J Clin Pharmacol 1995, 35(1): 87-97. 37. Weidmann, P. Differential effects of antihypertensive drugs on hypertension - Associated risk factors. Cardiology 1994, 85(S1): 78-83. 38. Hall, J.M. Bradykinin receptors: Pharmacologi cal properties and biological roles. Pharmacol Ther 1993, 56: 131-90. 39. Sunman, W., Sever, P.S. Nonangiotensin effects of angiotensin converting enzyme inhibi tors. Clin Sci 1993, 85: 661-70. 40. Linz, W., Henning, R., Scholkens, B.A. Role of angiotensin II receptor and converting enzyme inhibition in the progression and regression of cardiac hypertrophy in rats. J Hypertens 1992, 9(Suppl. 6): 400-1S. 41. Rhaleb, N.E., Yang, X.P., Scicli, A.G., Carretero, O.A. Role of kinins and nitric oxide in the antihypertrophic effect of ramipril. Hypertension 1994, 23(Pt. 2): 865-8. 42. Farhy, R.D., Carretero, O.A., Ho, K.L., Scicli, A.G. Role of kinins and nitric oxide in the effects of angiotensin converting enzyme inhibitors on neointima formation. Circ Res 1993, 72(6): 1202-10. 43. Barnes, J.M., Barber, P.C., Barnes, N.M. Char acterisation of angiotensin II receptor subtypes in human cerebellar membranes. Brit J Pharma col 1992, 106(Suppl.): 135P (Abst). 44. Prescott, M.F., Webb, R.L., Reidy, M.A. Angio tensin converting enzyme inhibitor versus angiotensin II, AT1 receptor antagonist. Amer J Pathol 1992, 139(6): 1291-6. 45. Hartman, J.C., Hullinger, T.G., Wall, T.M., Shebuski, R.J. Reduction of myocardial infarct size by ramiprilat is independent of angiotensin II synthesis inhibition. Eur J Pharmacol 1993, 234(2-3): 229-36. 46. Miki, T., Miura, T., Shamamoto, K., Urabe, K., Sakamoto, J., limura, O. Do angiotensin con verting enzyme inhibitors limit myocardial infarct size?Clin Exp Pharmacol Physiol 1993, 20(6): 429-34. MEDICAMENTOS DE ACTUALIDAD 47. Campbell, D J., Kladis, A., Duncan, A.M. Effects of converting enzyme inhibitors on angiotensin and bradykinin peptides. Hypertension 1994, 23(4): 439-49. 48. Wong, P.C., Hart, S.D., Zaspel, A., Chiu, AT., Smith, R.D., Timmermans, P.B.M.W.M. Functional studies of nonpeptide angiotensin II receptor subtype-specific ligands: DuP 753 (AII-1) and PD123177(AII-2). J Pharmacol Exp Ther 1990, 255(2): 584-92. 49. Cachofeiro, V., Sakakibara, T., Nasjletti, A. Kinins, nitric oxide, and the hypotensive effect of captopril and ramiprilat in hypertension. Hyper tension 1992, 19(2): 138-45. 50. Geppetti, P. Sensory neuropeptide release by bradykinin: Mechanisms and pathophysiological implications. Regul Pept 1993, 47: 1-23. 51. Ogilvie, R.I. Research report - Acute effects of captopril, DuP 532 and Ro 44-9375 on hemodynamics and total vascular capacitance in exper imental acute heart failure. Unpublished 1994. 52. Peach, M.J. Renin-angiotensin system: Bio chemistry and mechanisms of action. Physiol Rev 1977,57:313-70. 53. Phillips, M.I., Speakman, E.A., Kimura, B. Tis sue renin angiotensin systems. In: Cellular and Molecular Biology of the Renin Angiotensin Sys tem. Raizada, M.K., Phillips, M.I., Sumners, C. (Eds.). CRC Press, Inc., Boca Raton 1993, 97-130. 54. Husain, A. The chymase angiotensin system in humans. J Hypertens 1993, 11: 1155-9. 55. Furakawa, Y., Kishimoto, S., Nishikawa, K. (Takeda Chem. Inds., Ltd.). Hypotensive imidazole derivatives and hypotensive imidazole-5-acetic acid derivatives. US 4340598, US 4355040. 56. Wong, P.O., Chiu, A.T., Price, W.A., Thoolen, M.J.M.C., Carini, D.J., Johnson, A.L., Taber, R.I., Timmermans, P.B.M.W.M. Nonpeptide angiotensin II receptor antagonists. I. Pharma cological characterization of 2-n-butyl-4-chloro1-(2-chlorobenzyl)imidazole-5-acetic acid, sodium salt (S-8307). J Pharmacol Exp Ther 1988,247:1-7. 57. Bovy, P.R., Olins, G.M. Recent advances in nonpeptidic angiotensin II receptor antagonists. In: Current Drugs: Renin Angiotensin System. Cur rent Patents, Ltd. 1992, B17-34. 58. Buhlmayer, P. Angiotensin II antagonists: Pat ent activity since the discovery of DuP 753. Curr Opin Ther Pat 1992, 2(1): 1693-718. 59. Wong, P.C., Tam, S.W., Herblin, W.F., Timmer mans, P.B.M.W.M. Further studies on the selec tivity of DuP 753, a nonpeptide angiotensin II DRUGS OF TODAY Vol. 31, No. 7, 1995 receptor antagonist. Eur J Pharmacol 1991, 196:201-3. 60. Liu, E.C.K., Hedberg, A., Goldenberg, H.J., Har ris, D.N., Webb, M.L. DuP 753, the selective angiotensin II receptor blocker, is a competitive antagonist to human platelet thromboxane A2/prostaglandin H 2 (TP) receptors. Prostaglandins 1992, 44(2): 89-99. 61. Chretien, L, Guillemette, G., Regoli, D. Nonpeptide angiotensin receptor antagonists bind to tachykinin NK3 receptors of rat and guinea pig brain. Eur J Pharmacol 1994, 256(1): 73-8. 62. Shibouta, Y., Inada, Y, Ojima, M., Wada, T., Noda, M., Sanada, T., Kubo, K., Kohara, Y, Naka, T., Nishikawa, K. Pharmacological profile of a highly potent and long acting angiotensin II receptor antagonist [(2'-(1H-tetrazol-5-yl)biphenyl - 4 - yl)methyl] - 1H- benzimidazole - 7 - carboxylic acid (CV-11974), and its prodrug, (±)-i(cyclohexyloxycarbonyloxy)-ethyl 2-ethoxy-1 1(2' - (1H - tetrazol - 5 - yl)biphenyl - 4 - yl)methyl] - 1H - benzimidazole - 7 - carboxylate (TCV-116). J Pharmacol ExpTher 1993,266(1 ): 114-20. 63. Wienen, W., Mauz, A.B.M., VanMeel, J.C.A., Entzeroth, M. Different types of receptor interac tion of peptide and nonpeptide angiotensin llantagonists revealed by receptor binding and functional studies. Mol Pharmacol 1992, 41(6): 1081-8. 64. Criscione, L., Thomann, H., Whitebread, S., DeGasparo, M., Buhlmayer, P., Herold, P, Ostermayer, F, Kamber, B. Binding characteris tics and vascular effects of various angiotensin II antagonists. J Cardiovasc Pharmacol 1990, 16(Suppl.4):S56-9. 65. Rhaleb, N.E., Rouissi, N., Nantel, R, D'OrleansJuste, P., Regoli, D. DuP 753 is a specific antag onist for the angiotensin receptor. Hypertension 1991, 17:480-4. 66. Timmermans, P.B.M.W.M., Wong, PC., Chiu ; AT., Herblin, W.R Nonpeptide angiotensin II receptor antagonists. Trends Pharmacol Sci 1991, 12:55-62. 67. MacFadyen, R.J., Tree, M., Lever, A.F, Reid, J.L. Effects of the angiotensin II receptor antag onist losartan (DuP 753/MK 954) oh arterial blood pressure, heart rate, plasma concentra tions of angiotensin II and renin and the pressor response to infused angiotensin II in the salt deplete dog.Clin Sci 1992, 83: 549-56. 68. Chan, D.P., Sandok, E.K., Aarhus, L.L., Heublein, D.M., Burnett, J.C. Jr. Renal specific actions of angiotensin II receptor antagonism in the anesthetized dog. Amer J Hypertens 1992, 5:354-60. 487 69. ElAmrani, A.I.K., Menard, J., Gonzales, M.F, Michel, J.B. Effects of blocking the angiotensin II receptor, converting enzyme, and renin activ ity on the renal hemodynamics of normotensive guinea pigs. J Cardiovasc Pharmacol 1993, 22(2): 231-9. 70. DeGraaf, G.L, Pals, D.T., Couch, S.J., Lawson, J.A. Hormonal and cardiovascular effects of losartan (DuP 753), an angiotensin receptor antagonist, in nonhuman primates. J Pharmacol ExpTher 1993, 264(1): 6-66. 71. Smid, S.D., Hime, N.J., Frewin, D.B., Head, R.J. Losartan reverses the angiotensin II mediated facilitation of sympathetic nerve transmission in the perfused rat mesenteric preparation. Clin Exp Pharmacol Physiol 1992, Suppl. 21: 65 (Abst). 72. Balla, T, Baukal, A.J., Eng, S., Catt, K.J. Angio tensin II receptor subtypes and biological responses in the adrenal cortex and medulla. Mol Pharmacol 1991, 40: 401-6. 73. Tofovic, S., Pong, A., Jackson, E.K. Effects of angiotensin subtype 1 and subtype 2 receptor antagonists in normotensive versus hyperten sive rats. Hypertension 1991, 18(6): 774-82. 74. Mizuno, K., Niimura, S., Tani, M., Haga, H., Gomibuchi, T, Sanada, H., Fukuchi, S. Antihypertensive and hormonal activity of MK 954 in spontaneously hypertensive rats. Eur J Phar macol 1992,215:305-8. 75. Wong, PC., Price, W.A., Chiu, AT., Carini, D.J., Duncia, J.V., Johnson, A.L., Wexler, R.R., Tim mermans, P.B.M.W.M. Nonpeptide angiotensin II receptor antagonists: Studies with EXP 9270 and DuP 753. Hypertension 1990, 15: 823-34. 76. Blair-West, J.R., Burns, P., Denton, D.A., Ferraro, T, McBurnie, M.I., Tarjan, E., Weisinger, R.S. Thirst induced by increasing brain sodium concentration is mediated by brain angiotensin. Brain Res 1994, 637(1-2): 335-8. 77. Li, Z., Ferguson, A.V. Electrophysiological evi dence that subfornical organ efferents to the paraventricular nucleus utilize angiotensin as an excitatory neuretransmitter in the rat. Unpub lished 1993. 78. Matsuo, K., Kumagai, K., Onon, M., Yamanouchi, Y, Handa, K., Nakashima, Y, Arakawa, K. Protective effects of MK954 on reperfusion arr hythmias in the dog. Unpublished 1992. 79. Song, K., Zhuo, J., Mendelsohn, F.A.O. Access of peripherally administered DuP 753 to rat brain angiotensin II receptors. Brit J Pharmacol 1991, 104:771-2. 80. Smith, R.D., Timmermans, P.B.M.W.M. Human angiotensin receptor subtypes. Curr Opin Nephrol Hypertens 1994, 3: 112-22. 488 81. Timmermans, P.B.M.W.M., Smith, R.D. Angiotensin II receptor subtypes: Selective antago nists and functional correlates. Eur Heart J 1994, 15(Suppl. D): 79-87. 82. Timmermans, P.B.M.W.M., Wong, P.C., Chiu, AT., Smith, R.D. The preclinical basis of the therapeutic evaluation of losartan. J Hypertens 1995, 13(Suppl. 1): S1-13. 83. Goldberg, A.I., Dunlay, M.C., Sweet, C.S. Safety and tolerability of losartan potassium, an angiotensin II receptor antagonist, compared with hydrochlorothiazide, atenolol, felodipine ER, and angiotensin-converting enzyme inhibi tors for the treatment of systemic hypertension. Amer J Cardiol 1995, 75(12): 793-5. 84. Inagami, T, Guo, D.F., Kitami, Y. Molecular biol ogy of angiotensin II receptors: An overview. J Hypertens 1994, 12(S10): S83-94. 85. Veltmar, A., Culman, J., Qadri, F, Rascher, W., Unger, T. Involvement of adrenergic and angiotensinergic receptors in the paraventricular nucleus in the angiotensin II induced vasopressin release. J Pharmacol Exp Ther 1992, 263(3): 1253-74. 86. Toney, G.M., Porter, J.P. Functional role of brain AT1 andAT 2 receptors in the central angiotensin II pressor response. Brain Res 1993, 603(1): 57-63. 87. Toney, G.M., Porter, J.P. Functional roles of brain AT1 andAT 2 receptors in the central angio tensin II pressor response in conscious young spontaneously hypertensive rats. Dev Brain Res 1993, 71: 193-9. 88. Hogarty, D.C., Speakman, E.A., Puig, V., Phil lips, M.I. The role of angiotensin, AT 1 and AT 2 receptors In the pressor, drinking and vasopressin responses to central angiotensin. Brain Res 1992, 586(2): 289-94. 89. Rippetoe, P.E., Fitz, R., Painter, D., Soltis, E., Gillespie, M.N., Cassis, L. Angiotensin (AII) and monocrotaline (MCT)-induced pulmonary hypertension: Effect of DuP 753, a nonpeptide AII-1 receptor antagonist. FASEB J 1991, 5(4, Pt. I): A535 (Abst). 90. Rump, L.C., Schwertfeger, E., Schaible, U., Fraedrich, G., Schollmeyer, P. 2 Adrenergic receptor and angiotensin II receptor modulation of sympathetic neurotransmission in human atria. Circ Res 1994, 74(3): 434-40. 91. Foucart, S., Patrick, S.K., Oster, L., DeChamplain, J. Effects of chronic treatment with losar tan and enalaprilat on [ 3 H]-noradrenaline from isolated atria of WKY and SHR rats. Unpub lished 1994. MEDICAMENTOS DE ACTUALIDAD 92. Rump, L.C. Angiotensin (A) II receptor blockade and neurotransmission in human kidney cortex. J Hypertens 1994, 12(Suppl. 3): 868 (Abst). 93. Niederberger, M., Nussberger, J., Brunner, H.R., Waeber, B. Effects of ACE inhibition, angiotensin II receptor blockade and Na-nitroprusside on sympathetic nerve activity of renal hypertensive rats. Circulation 1990, 82(4): III-684 (Abst). 94. Wong, PC., Bernard, R., Timmermans, P.B.M.W.M. Effect of blocking angiotensin II receptor subtype on rat sympathetic nerve function. Hypertension 1992, 19(6, Pt. 2): 663-7. 95. Hilgers, K.F., Veelken, R., Ganten, D., Luft, F.C., Geiger, H., Mann, J.F.E. Vascular angiotensin formation and the sympathetic nervous system in Ren-2 transgenic hypertensive and control rats. Hypertension 1993, 22(3): 424 (Abst). 96. Schwieler, J.H., Kahan, T, Nussberger, J., Hjemdahl, P. Converting enzyme inhibition mod ulates sympathetic neurotransmission in vivo via multiple mechanisms. Amer J Physiol 1993, 264(27): E631-7. 97. Wong, PC., Price, W.A., Chiu, A.T., Duncia, J.V., Carini, D.J., Wexler, R.R., Johnson, A.L., Tim mermans, P.B.M.W.M. Nonpeptide angiotensin II receptor antagonists. IX. Antihypertensive activity in rats of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 1990,252(2): 726-32. 98. Ling, Q., Guo, Z.G., Su, Z., Guo, X. Regression of cardiac hypertrophy and myosin isoenzyme patterns by losartan and captopril in renovascular hypertensive rats. Acta Pharmacol Sin 1994, 15(3): 206-10. 99. Wilm, C., Lues, I., Schelling, P. Regression of vascular hypertrophy in renal hypertensive rats treated with the angiotensin II antagonist losar tan. J Hypertens 1994, 12(Suppl. 3): S90. 100. Schudeck, R., Jalil, J.E., Vio, C., Nalli, A., Cespedes, C., Ebensperger, R. Regression of renal fibrosis and damage in experimental renovascular hypertension using an angiotensin type 1 receptor antagonist. J Amer Coll Cardiol 1994, Special Issue: 332A (Abst). 101. Tabrizchi, R., Lupichuk, S.M. The effects of losartan and captopril on vasopressor actions of cirazoline in the absence and presence of SZL-49 and nifedipine. J Cardiovasc Pharmacol 1995,26(1): 137-44. 102. Bunkenburg, B., Schnell, C., Baum, H.P, Cumin, F, Wood, J.M. Prolonged angiotensin II antagonism in spontaneously hypertensive rats DRUGS OF TODAY Vol. 31, No. 7, 1995 - Hemodynamic and biochemical consequences. Hypertension 1991, 18(3): 278-88. 103. Soltis, E.E., Jewell, A.L., Dwoskin, L.P., Cassis, L.A. Acute and chronic effects of losartan (DuP 753) on blood pressure and vascular reactivity in normotensive rats. Clin Exp Hypertension 1993, 15(1): 171-84. 104. Wong, P.C., Hart, S.D., Duncia, J.V., Timmermans, P.B.M.W.M. Nonpeptide angiotensin II receptor antagonists. XIII. Studies with DuP 753 and EXP3174 in dogs. Eur J Pharmacol 1991, 202: 323-30. 105. Doig, J.K.,MacFadyen,R.J., Sweet, C.S., Reid, J.L. Hemodynamic and renal responses to oral losartan potassium during salt depletion in nor mal human volunteers. J Cardiovasc Pharmacol 1995, 25(1): 511-7. 106. Wong, P.C., Price, W.A., Chiu, AT, Duncia, J.V., Carini, D.J., Wexler, R.R., Johnson, A.L., Timmermans, P.B.M.W.M. Pharmacology of EXP3174, a metabolite of DuP 753, an orally active nonpeptide angiotensin II (All) receptor antagonist. J Hypertens 1990, 8(Suppl. 3): S42 (Abst). 107. McMahon, T.J., Kaye, A.D., Hood, J.S., Minkes, R.K., Nossaman, B.D., Kadowitz, P.J. Inhibitory effects of DuP 753 and EXP3174 on responses to angiotensin II in pulmonary vascular bed of the cat. J Appl Physiol 1992, 73(5): 2054-61. 108. Chiu, AT., Leung, K.H., Smith, R.D., Timmer mans, P.B.M.W.M. Defining angiotensin recep tor subtypes. In: Cellular and Molecular Biology of the Renin Angiotensin System. Raizada, M.K., Phillips, M.I., Sumners, C. (Eds.). CRC Press, Inc., Boca Raton 1993, 245-71. 109. Christ, D.D., Wong, PC., Wong, Y.N., Hart, S.D., Quon, C.Y., Lam, G.N. The pharmacokinetics and pharmacodynamics of the angioten sin II receptor antagonist losartan potassium (DuP 753/MK954) in the dog. J Pharmacol Exp Ther 1994, 268(3): 1199-205. 110. Stearns, R.A., Miller, R.R., Doss, G.A., Chakravarty, P.K., Rosegay, A., Gatto, G.J., Chiu, S.H.L. The metabolism of DuP 753, a nonpep tide angiotensin II receptor antagonist, by rat, monkey and human liver slices. Drug Metab Dispos 1992, 20(2): 281-7. 111. Krieter, P.A., Colletti, A.E., Miller, R.R,, Stearns, R.A. Absorption and glucuronidation of the angiotensin II receptor antagonist losartan by the rat intestine. J Pharmacol Exp Ther 1995, 273(2): 816-22. 112. Christen, Y, Waeber, B., Nussberger, J., Porchet, M., Lee, R., Maggon, K., Shum, L., Tim mermans, P.B.M.W.M., Brunner, H.R., Borland, R.M. Oral administration of DuP 753, a specific 489 angiotensin II antagonist, to normal male volunteers: Inhibition of pressor response to exogenous angiotensin I and II. Circulation 1991, 83(4): 1333-42. 113. Timmermans, P.B.M.W.M., Chiu, AT., Herblin, W.F., Wong, P.C., Smith, R.D. Angiotensin II receptor subtypes. Amer J Hypertens 1992, 5: 406-10. 114. Timmermans, P.B.M.W.M., Benfield, P., Chiu, AT., Herblin, W.F., Wong, P.C., Smith, R.D. Angiotensin II receptors and functional corre lates. Amer J Hypertens 1992, 5: 221-35S. 115. Chiu, AT., Herblin, W.F., Ardecky, R.J., McCall, D.E., Carini, D.J., Duncia, J.V., Pease, L.J., Wexler, R.R., Wong, P.C., Johnson, A.L., Tim mermans, P.B.M.W.M. Identification of angio tensin II receptor subtypes. Biochem Biophys Res Commun 1989, 165(1): 196-203. 116. Whitebread, S., Mele, M., Kamber, B., DeGasparo, M. Preliminary biochemical characteriza tion of two angiotensin II receptor subtypes. Bio chem Biophys Res Commun 1989,163:284-91. 117. Bumpus, F.M., Catt, K.J., Chiu, AT., DeGasparo, M., Goodfriend, T, Husain, A., Peach, M.J., Taylor, D.G. Jr., Timmermans, P.B.M.W.M. Nomenclature for angiotensin receptors. Hyper tension 1991, 17(5): 720-1. 118. DeGasparo, M., Husain, A., Alexander, W., Catt, K.J., Chiu, AT, Drew, M., Goodfriend, T, Harding, J.W., Inagami, T., Timmermans, P.B.M.W.M. Proposed update of the angiotensin receptor nomenclature. Hypertension 1995, 25(5): 924-7. 119. Cazaubon, C., Gougat, J., Bousquet, F., Guiraudou, P., Gayraud, R., Lacour, C., Roccon, A., Galindo, G., Barthelemy, G. Pharmacological characterization of SR 47436, a new nonpeptide AT1 subtype angiotensin II receptor antagonist. J Pharmacol Exp Ther 1993, 265(2): 826-34. 120. Criscione, L., DeGasparo, M., Buhlmayer, P., Whitebread, S., Ramjoue, H.P., Wood, J.M. Pharmacological profile of valsartan: A potent, orally active, nonpeptide antagonist of the angiotensin II AT1 receptor subtype. Brit J Phar macol 1993, 110(2): 761-71. 121. Catt, K.J., Sandberg, K., Balla, T. Angiotensin II receptors and signal transduction mechanisms. In: Cellular and Molecular Biology of the Renin Angiotensin System. Raizada, M.K., Phillips, M.I., Sumners, C. (Eds.). CRC Press, Inc., Boca Raton 1993, 307-56. 122. Macari, D., Bottari, S.P., Whitebread, S., DeGasparo, M., Levens, N. Renal actions of the selective angiotensin AT2 receptor ligands CGP 42112B and PD 123319 in the sodium depleted rat. Eur J Pharmacol 1993, 249(1): 85-93. 490 123. Chiu, AT., McCall, D.E., Ardecky, R.J., Duncia, J.V., Nguyen, T.T., Timmermans, P.B.M.W.M. Angiotensin. II receptor subtypes and their selective nonpeptide ligands. Receptor 1990,1: 33-40. 124. Crawford, K.W., Frey, E.A., Cote, T.E. Angiotensin II receptor recognized by DuP753 regulates two distinct guanine nucleotide-binding protein signaling pathways. Mol Pharmacol 1992, 41(1): 154-62. 125. Ohyama, K., Yamano, Y, Chaki, S., Kondo, T., Inagami, T. Domains for G-protein coupling in angiotensin II receptor type 1: Studies by site-di rected mutagenesis. Biochem Biophys Res Commun 1992, 189(2): 677-83. 126. Wintersgiil, H.P., Warburton, P., Bryson, S.E., Ball, S.G., Balmforth, A.J. Characterization of the angiotensin II receptor expressed by the human hepatoma cell line, PLC-PRF-5. Eur J Pharmacol 1992, 227: 283-91. 127. Pfeilschifter, J., Huwiler, A., Merriweather, C., Briner, V.A. Angiotensin II stimulation of phospholipase D in rat renal mesangial cells is mediated by the AT 1 receptor subtype. Eur J Pharmacol 1992, 225(1): 57-62. 128. Pfeilschifter, J. Angiotensin II B-type receptor mediates phosphoinositide hydrolysis in mesangial cells. Eur J Pharmacol 1990, 184: 201-2. 129. Ko, Y, Gorg, A., Appenheimer, M., Wieczorek, A.J., Dusing, R., Vetter, H., Sachinidis, A. Losartan inhibits the angiotensin II induced stimula tion of the phosphoinositide signaling system in vascular smooth muscle cells. Eur J Pharmacol 1992, 227(2): 215-9. 130. Poggioli, J., Lazar, G., Houillier, P., Gardin, J.P., Achard, J.M., Paillard, M. Effects of angiotensin II and nonpeptide receptor antagonists on the transduction pathways in rat proximal tubule. Amer J Physiol 1992, 263(32): C750-8. 131. Clyne, C.D., Nicol, M.R., MacDonald, S., Wil liams, B.C., Walker, S.W. Angiotensin II stimu lates growth and steroidogenesis in zona fasciculata/reticularis cells from bovine adrenal cortex via the AT1 receptor subtype. Endocrinol ogy 1993, 132(5): 2206-12. 132. Hajnoczky, G., Csordas, G., Hunyady, L, Kalapos, M.P., Balla, T., Enyedi, P., Spat, A. Angio tensin II inhibits sodium/potassium pump in rat adrenalglomerulosa cells: Possible contribution to stimulation of aldosterone production. Endo crinology 1992, 130(3): 1637-44. 133. Bunkenburg, B., VanAmelsvoort, T., Rogg, H., Wood, J.M. Receptor mediated effects of angio tensin II on growth of vascular smooth muscle MEDICAMENTOS DE ACTUALIDAD cells from spontaneously hypertensive rats. Hypertension 1992, 20(6): 746-54. 134. Keiser, J.A., Bjork, F.A., Hodges, J.C., Taylor, D.G. Jr. Renal hemodynamic and excretory responses to PD123319 and losartan, nonpep tide AT1 and AT2 subtype specific angiotensin II ligands. J Pharmacol Exp Ther 1992, 262(3): 1154-60. 135. Macari, D., Whitebread, S., Cumin, R, DeGasparo, M., Levens, N. Renal actions of the angio tensin AT 2 receptor ligands CGP42112 and PD 123319 after blockade of the renin angioten sin system. Eur J Pharmacol 1994, 259(1): 27-36. 136. Stephenson, K.N., Steele, M.K. Brain angioten sin II receptor subtypes and the control of luteinizing hormone and prolactin secretion in female rats. J Neuroendocrinol 1992, 4(4): 441-7. 137. Mukoyama, M., Nakajima, M., Horiuchi, M., Sasamura, H., Pratt, R.E., Dzau, V.J. Expres sion cloning of type 2 angiotensin II receptor reveals a unique class of seven transmembrane receptors. J Biol Chem 1993, 268(33): 24539-42. 138. Kambayashi, Y, Bardhan, S., Takahashi, K., Tsuzuki, S., Inui, H., Hamakubo, T., Inagami, T. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem 1993, 268(33): 24543-6. 139. Cox,B.E., Ipson, M.A., Shaul, P.W., Kamm, K.E., Rosenfeld, C.R. Myometrial angiotensin II receptor subtypes change during ovine preg nancy. J Clin Invest 1993, 92: 2240-8. 140. Cox, B.E., Williams, C.E., Roy, T.A., Rosenfeld, C.R. Angiotensin II (ANG II) may not directly vasoconstrict the uteroplacental circulation in pregnant ewes. FASEB J 1995, 9(4): A896 (Abst). 141. Grady, E.F., Kalinyak, J.E. Expression of AT2 receptors in rat fetal subdermal cells. Regul Pept 1993, 44(2): 171-80. 142. Kang, J., Sumners, C., Posner, P. Angiotensin II type 2 receptor modulated changes in potas sium currents in cultured neurons. Amer J Physiol 1993, 265(3): C607-16. 143. Kang, J., Posner, P., Sumners, C. Angiotensin II type 2 receptor stimulation of neuronal K + cur rents involves an inhibitory GTP binding protein. Amer J Physiol 1994, 267(5, Pt. 1): C1389-97. 144. Morishita, R., Gibbons, G.H., Ellison, K.E., Lee, W., Zhang, L., Yu, H., Kaneda, Y, Ogihara, T., Dzau, V.J. Evidence for direct local effect of angiotensin in vascular hypertrophy in vivo gene DRUGS OF TODAY Vol. 31, No. 7, 1995 transfer of angiotensin converting enzyme. J Clin Invest 1994, 94(3): 978-84. 145. Kauffman, R.F., Bean, J.S., Zimmerman, K.M., Brown, R.F., Steinberg, M.I. Losartan, a nonpeptide angiotensin II (ANG II) receptor antago nist, inhibits neointima formation following bal loon injury to rat carotid arteries. Life Sci 1991, 49(25): PL223-8. 146. Bourgeois, R., Laporte, S., Escher, E. The myoproliferative response of vascular smooth muscle after injury is angiotensin II mediated through the AT1 receptor. FASEB J 1993, 7(3): A47 (Abst). 147.Huckle, W.R., Drag, M.D., Acker, W.R., Powers, M., Holder, D.J., Kim, D., Mantlo, N.B., Greenlee, W.J., Johnson, R.G. Neither an AT1 -selec tive nor a balanced AT 1/AT 2 angiotensin II receptor antagonist reduces neointimal thicken ing in a porcine coronary artery injury model. Unpublished 1994. 148. Stoll, M., Steckelings, U.M., Paul, M., Bottari, S.P., Metzger, R., Unger, T. The angiotensin AT2-receptor mediates inhibition of cell prolifer ation in coronary endothelial cells. J Clin Invest 1995,95:651-7. 149. Okunishi, H., Shiota, N., Fukamizu, A., Takai, S., Murakaml, K., Miyazaki, M. Angiotensin II antagonist, non ACE inhibitor, prevents neointima formation on injured canine arteries. J Hypertens 1994, 12(Suppl. 3): Abst 725. 150. Wong, P.C. Angiotensin antagonist in models of hypertension. In: Angiotensin Receptors. Saavedra, J.M., Timmermans, P.B.M.W.M. (Eds.). Plenum Press, New York 1994, 319-36. 151. Dostal, D.E., Baker, K.M. Angiotensin II stimula tion of left ventricular hypertrophy in adult rat heart. Amer J Hypertens 1992, 5: 276-80. 152. Everett, A.D., Tufro-McReddie, A., Fisher, A,, Gomez, R.A. Angiotensin receptor regulates cardiac hypertrophy and transforming growth factor 1 expression. Hypertension 1994, 23(5): 587-92. 153. Bruckschlegel, G., Holmer, S.R., Jandeleit, K., Ackermann, B., Riegger, G.A.J, Schunkert, H. The role of cardiac angiotensin conversion enzyme in experimentally induced myocardial hypertrophy in the rat. Nier Hochdruckkrank 1994, 23(4): 144-6. 154. Wong, P.C., Price, W.A., Chiu, AT., Duncia, J.V., Carini, D.J., Wexler, R.R., Johnson, A.L., Timmermans, P.B.M.W.M. In vivo pharmacol ogy of DuP 753. Amer J Hypertens 1991, 4(4): 288-98S. 155. Morton, J. J. Inhibiting the effects of angiotensin II on cardiovascular hypertrophy in experimen tal hypertension. In: Angiotensin Receptors. 491 Saavedra, J.M., Timmermans, P.B.M.W.M. (Eds.). Plenum Press, New York 1994, 221-34. 156. Soitis, E.E. Alterations in vascular structure and function after short term losartan treatment in spontaneously hypertensive rats. J Pharmacol Exp Ther 1993, 266(2): 642-6. 157. Ganten, D., Schelling, P., Flugel, R.M., Fischer, H. Effect of angiotensin and the angiotensin antagonist P113 on iso-renin and cell growth in 3T3 mouse cells. IRCS Med Sci Biochem 1975, 3:327. 158. Huckle, W.R., Earp, H.S. Regulation of cell pro liferation and growth by angiotensin II. Prog Growth Factor Res 1994, 5(2): 177-94. 159. Wolf, G. Regulation of renal tubular cell growth: Effects of angiotensin II. Exp Nephrol 1994, 2(2): 107-14. 160. Wu, J.N., Edwards, D., Berecek, K.H. Changes in renal angiotensin II receptors in sponta neously hypertensive rats by early treatment with the angiotensin converting enzyme inhibi tor captopril. Hypertension 1994, 23(Pt 2): 819-22. 161. VonLutterotti, N., Camargo, M.J.F., Mueller, F.B., Timmermans, P.B.M.W.M., Laragh, J.H. Angiotensin II receptor antagonist markedly reduces morbidity in salt-loaded Dahl S rats. Amer J Hypertens 1991, 4(4, Pt. 2): 346-9S. 162. Camargo, M.J.F., VonLutterotti, N., Pecker, M.S., James, G.D., Timmermans, P.B.M.W.M., Laragh, J.H. DuP 753 increases survival in spontaneously hypertensive stroke-prone rats fed a high sodium diet. Amer J Hypertens 1991, 4(4, Pt. 2):341-5S. 163. Beinlich, C.J., Morgan, H.E. Control of growth in neonatal pig hearts. Mol Cell Biochem 1993, 119(1-2): 3-9. 164. Milavetz, J.J., Raya, T.E., Morkin, E., Goldman, S. Survival after large myocardial infarction in rats: Comparison of ACE inhibition with captopril versus direct angiotensin II blockade with losartan. 44th Annu Sci Sess Amer Coll Cardiol 1994. 165. Gonzalez-Espinosa, C., Garcia-Sainz, J.A. Angiotensin II and active phorbol esters induce proto-oncogene expression in isolated rat hepatocytes. Biochim Biophys Acta 1992, 1136: 309-14. 166. Lyall, F., Dornan, E.S., McQueen, J., Boswell, F, Kelly, M. Angiotensin II increases protooncogene expression and phosphoinositide turnover in vascular smooth muscle cells via the angiotensin II AT1 receptor. J Hypertens 1992, 10(12): 1463-70. 167. Millet, D., Desgranges, C., Campan, M., Gadeau, A., Costerousee, O. Effects of angio- 492 tensins on cellular hypertrophy and c-fos expression in cultured arterial smooth muscle cells. Eur J Biochem 1992, 206(2): 367-72. 168. Rydzewski, B., Wozniak, M., Baker, S.P.,Sumners, C., Raizada, M.K. Angiotensin II (All) stimulation of c-fos gene expression in neuronal and astroglial cultures from the brain. Hypertension 1992, 20(3): 412 (Abst). 169. Schorb, W., Booz, G.W., Dostal, D.E., Chang, K.C., Baker, K.M. Angiotensin II receptor mediated growth of cardiac fibroblasts. Circula tion 1992, 86(4): 1-89 (Abst). 170. Viard, l, , Jaillard, C., Ouali, R., Saez, J.M. Angiotensin II induced expression of proto oncogene (c-fos, jun-B and c-jun) mRNA in bovine adrenocortical fasciculata cells (BAC) is mediated by AT 1 receptors. FEBS Lett 1992, 313(1): 43-6. 171. Chua, B.H.L., Chua, C.C., Diglio, C.A., Siu, B.B. Induction of TGF-beta 1 by angiotensin II in rat heart endothellal cells. FASEB, J 1993, 7(3): A133 (Abst). 172. Scheuer, DA, Kitzen, J.M., Perrone, M.H. Role of nitric oxide release in the arterial pressure response to angiotensin. FASEB J 1993, 7(4): A765 (Abst). 173. Chiu, AT., Roscoe, W.A., McCall, D.E., Timmermans, P.B.M.W.M. The type of angiotensin II receptors found in rat, aortic smooth muscle cells. J Hypertens 1990, 8(Suppl. 3): 167 (Abst). 174. Sachinidis, A., Ko, Y., Weisser, P., Brickwedde, M.K.M.Z., Dusing, R., Christian, R., Wieczorek, A.J., Vetter, H. EXP3174, a metabolite of losartan (MK-954, DuP 753) is more potent than losartan in blocking the angiotensin II induced responses in vascular smooth muscle cells. J Hypertens 1993, 11(2): 155-62. 175. Anderson, P.W., Do, Y.S., Hsueh, W.A. Angio tensin II causes mesangial cell hypertrophy. Hypertension 1993, 21(1): 29-35. 176. Chansel, D., Badre, L., Czekalski, S. Intrinsic properties of the nonpeptide angiotensin II (All) antagonist losartan in glomeruli and mesangial cells (MC) at high concentrations. J Amer Soc Nephrol 1992, 3(3): 434 (Abst), 177. DeLaGarza, M., Bhandaru, S., Bakris, G.L. Angiotensin II (All) induced mitogenesis in human mesangial cells (HMO): Role for endothelin. J Amer Soc Nephrol 1992, 3(3): 466 (Abst). 178. Kessler-lcekson, G., Schlesinger, H., Cohen, F. Effect of angiotensin II and losartan on protein accumulation in cultured heart myocytes and non-myocytes. FASEB J 1992, 6(5): A1872 (Abst). MEDICAMENTOS DE ACTUALIDAD 179. Schunkert, H., Weinberg, E.G., Izumo, S., Riegger, G., Lorell, B.H. Angiotensin II mediated rapid increase in cardiac protein synthesis in adult hearts is not associated with induction of c-fos, c-jun, and c-myc protooncognees. FASEB J 1993, 7(4): A751 (Abst). 180. Wamsley, J.K., Herblin, W.F., Hunt, M. Use of nonpeptide angiotensin H receptor antagonists to demonstrate All receptor subtypes in brain. Soc Neuro Sci 1990, 16(1): 685 (Abst). 181. Holycross, B.J., Peach, M.J., Owens, G.K. Angiotensin II stimulates increased protein syn thesis, not increased DNA synthesis, in intact rat aortic segments, in vitro. J Vase Res 1993, 30: 80-6. 182. Madhun, Z., Ernsberger, P., Ke, F.C., Zhou, J., Hopfer, U., Douglas, J.G. Signal transduction mediated by angiotensin II receptor subtypes expressed in rat renal mesangial cells. Regul Pept 1993,44(2): 149-57. 183. Beinlich, C.J., White, G.J., Baker, K.M., Mor gan, H.E. Angiotensin II and left ventricular growth in newborn pig heart. J Mol Cell Cardiol 1991 , 23(9): 1031-8. 184. Morton, J.J., Beattie, E.G., MacPherson, F. Angiotensin II receptor antagonist losartan has persistent effects on blood pressure in the young spontaneously hypertensive rat: Lack of relation to vascular structure. J Vase Res 1992, 29(3): 264-9. 185. Lafayette, R.A., Mayer, G., Park, S.K., Meyer, T.W. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest 1992, 90(3): 766-71. 186. Pollock, D.M., Divish, B.J., Polakowski, J.S., Opgenorth, T.J. Angiotensin II receptor block ade improves renal function in rats with reduced renal mass. J Pharmacol Exp Ther 1993, 267(2): 657-63. 187. Remuzzi, A., Perico, N., Amuchastegui, C.S., Malanchini, B., Mazerska, M., Battaglia, C., Bertani, T., Remuzzi, G. Short and long term effect of angiotensin II receptor blockade in rats with experimental diabetes. J Amer Soc Nephrol 1993,4:40-9. 188. Ruzicka, M., Yuan, B., Harmsen, E., Leenen, F.H.H. The renin angiotensin system and vol ume overload-induced cardiac hypertrophy in rats. Circulation 1993, 87(3): 921-30. 189. Raya, T.E., Fonken, S.J., Lee, R.W., Daugherty, S., Goldman, S., Wong, P.C., Timmermans, P.B.M.W.M., Morkin, E. Hemodynamic effects of direct angiotensin II blockade compared to con-. verting enzyme inhibition in rat model of heart DRUGS OF TODAY Vol. 31, NO. 7, 1995 failure. Amer J Hypertens 1991, 4(4, Pt. 2): 334-40S. 190. Capasso, J.M., Li, P., Meggs, L.G., Herman, M.V., Anversa, P. Efficacy of angiotensin con verting enzyme inhibition and AT 1 receptor blockade on cardiac pump performance after myocardial infarction in rats. J Cardiovasc Pharmacol 1994, 23(4): 584-93. 191. Okada, M., Kobayashi, M., Satoh, N., Nishikibe, M., Ikemoto, F. Effect of chronic treatment with losartan on development of hypertension in stroke prone spontaneously hypertensive rats (SHRSP): Comparative study with enalapril and hydralazine. Hypertens Res 1993, 16: 49-55. 192. Chang, R.S.L., Lotti, V.J. Angiotensin receptor subtypes in rat, rabbit and monkey tissues: Rel ative distribution and species dependency. Life Sci 1991,49(20): 1485-90. 193. Grone, H.J., Simon, M., Fuchs, E. Autoradiographic characterization of angiotensin receptor subtypes in fetal and adult human kidney. Amer J Physiol 1992, 262(2): F326-31. 194. Burns, K.D., Homma, T., Harris, B.C. The intrarenal renin angiotensin system. Semin Nephrol 1993, 13(1): 13-30. 195. Hall, J.E., Guyton, A.C., Brands, M.W. Control of sodium excretion and arterial pressure by intrarenal mechanisms and the renin angiotensin system. In: Hypertension: Pathophysiology, Diagnosis, and Management, 2nd Ed. Laragh, J.H., Brenner, B.M. (Eds.). Raven Press, New York 1995, 1451-75. 196. Fenoy, F.J., Milicic, I., Smith, R.D., Wong, P.C., Timmermans, P.B.M.W.M., Roman, R.J. Effects of DuP 753 on renal function of normotensive and spontaneously hypertensive rats. Amer J Hypertens 1991, 4(4, Pt. 2): 321-6S. 197. Schmieder, R.E. Nephroprotection by antihypertensive agents. J Cardiovasc Pharmacol 1994, 24(Suppl.2):S55-64. 198. Tanaka, R., Kon, V., Yoshioka, T., Ichikawa, I., Fogo, A. Angiotensin converting enzyme inhibi tor modulates glomerular function and structure by distinct mechanisms. Kidney Int 1994, 45: 537-43. 199. Jover, B., Mimran. A. Angiotensin II receptor antagonists versus angiotensin converting enzyme inhibitors: Effects on renal function. J Hypertens 1994, 12(9): 3-9S. 200. Levine, D.Z., lacovitti, M., Buckman, S., Burns, K. D. Response of rat late distal tubule bicarbon ate flux to changes in the renin angiotensin sys tem in vivo. Unpublished 1995. 201. Tobe, T.J.M., deLangen, C.D.J, Weersink, E.G.L, Wijngaarden, J.V., Bel, K.J., deGraeff, P.A., Gilst, W.H.V., Wesseling, H. The angioten- 493 sin converting enzyme inhibitor perindopril improves survival after experimental myocardial infarction in pigs. J Cardiovasc Pharmacol 1992, 19:732-40. 202. Camargo, M.J.F., VonLutterotti, N., Campbell, W.G., Pecker, M.S., James, G.D., Timmermans, P.B.M.W.M. Control of blood pressure and end organ damage in maturing salt loaded stroke prone spontaneously hypertensive rats by oral angiotensin II receptor blockade. J Hypertens 1993, 11(1): 31-40. 203. Honma, H., Nakamura, T., Ikeda, Y., Kuroyanagi, R., Takano, H., Yoshida, Y, Tamura, K. Renal protective effects of the angiotensin // antagonist CV-11974 in spontaneously hyper tensive rats stroke prone. J Hypertens 1994, 12(Suppl. 3): 2075 (Abst). 204. Saladini, D., Jover, B., Ribstein, J. Renal com pensation to uninephrectomy (UNX) and the renin angiotensin system. J Amer Soc Nephrol 1992, 3(3): 478 (Abst). 205. Jackson, E.K., Inagami, T. Blockade of the preand postjunctional effects of angiotensin in vivo with a nonpeptide angiotensin receptor antago nist. Life Sci 1990, 46: 945-53. 206. Yayama, K., Kawao, M., Tujii, H., Itoh, N., Okamoto, H. DuP 753 prevents the development of puromycin aminonucleoside induced nephrosis. Eur J Pharmacol 1993, 236: 337-8. 207. Pimentel, J.L., Wang, S., Martinez-Maldonado, M. Regulation of the renal angiotensin II recep tor gene in acute unilateral ureteral obstruction. Kidney Int 1994, 45(6): 1614-21. 208. Gekle, M., Silbernagl, S. Mechanism of ochratoxin A induced reduction of glomerular filtration rate in rats. J Pharmacol Exp Ther 1993, 267(1): 316-21. 209. Sakemi, T., Baba, N. Effects of an angiotensin II receptor antagonist on the progression of renal failure in hyperlipidemic Imai rats. Nephron 1993, 65(3): 426-32. 210. Hutchison, F.N. Hormonal modulation of proteinuria in the nephrotic syndrome. Amer J Neph rol 1993, 13(5): 337-46. 211. Yayama, K., Matsui.T., Takano, M., Hayashi, K., Nagamatsu, T., Suzuki, Y, Okamoto, H. Activa tion of the renin-angiotensin system in antiglomerular basement membrane antibody induced glomerulonephritis. Biol Pharm Bull 1995,18(3): 411-5. 212. Smith, R.D., Timmermans, P.B.M.W.M. Inhibi tion of the renin angiotensin system in conges tive heart failure. Curr Drugs 1992, A127-A150. 213. Baker, K.M., Booz, G.W., Dostal, D.E. Cardiac actions of angiotensin H: Role of an intracardiac 494 renin angiotensin system. Annu Rev Physiol 1992,54:227-41. 214. Johnston, C.I. Tissue angiotensin converting enzyme in cardiac and vascular hypertrophy, repair, and remodeling. Hypertension 1994, 23(2): 258-68. 215. Urata, H., Ganten, D. Cardiac angiotensin II formation: The angiotensin I converting enzyme and human chymase. Eur Heart J 1993, 14(Suppl. I): 177-82. 216. Urata, H., Hoffmann, S., Ganten, D. Tissueangiotensin II system in the human heart. Eur Heart J 1994, 15(D): 68-78. 217. Brasch, H., Sieroslawski, L, Dominiak, P. Angiotensin II increases norepinephrine release from atria by acting on angiotensin subtype 1 receptors. Hypertension 1993, 22: 699-704. 218. Foucart, S., Patrick, S.K., Oster, L., DeChamplain, J. Effect of chronic treatment with losartan and enalaprilat on [3H]-noradrenaline release from isolated atria of SHR rats. J Hypertens 1994, 12(Suppl. 3): 488 (Abst). 219. Scott, A.L., Chang, R.S.L., Lotti, V.J., Siegl, P.K.S. Cardiac angiotensin receptors: Effects of selective angiotensin II receptor antagonists, DuP 753andPD121981, in rabbit heart. J Pharmacol Exp Ther 1992, 261(3): 931-5. 220. Ishihata, A., Endoh, M. Pharmacological char acteristics of the positive inotropic effect of angiotensin II In the rabbit ventricular myocar dium. Brit J Pharmacol 1993, 108: 999-1005. 221. Zerkowski, H.R., Broede, A., Kunde, K., Hillemann, S., Schafer, E., Vogelsang, M., Michel, M.C., Brodde, O.E. Comparison of the positive inotropic effects of serotonin, histamine, angio tensin H, endothelin and isoprenaline in the iso lated human right atrium. Naunyn-Schmied Arch Pharmacol 1993, 347: 347-52. 222. Holubarsch, C., Hasenfuss, G., SchmidtSchweda, S., Knorr, A., Pieske, B., Ruf, T., Fasol, R., Just, H. Angiotensin I and II exert inotropic effects in atrial but not in ventricular human myocardium. Circulation 1993, 88: 1228-37. 223. Weber, K.T., Sun, Y., Guarda, E. Structural remodeling in hypertensive heart disease and the role of hormones. Hypertension 1994,23(Pt. 2): 869-77. 224. Guarda, E., Myers, P.R., Brilla, C.G., Tyagi, S.C., Weber, K.T. Endothelial cell induced mod ulation of cardiac fibroblast collagen metabo lism. Cardio Res 1993, 27(6): 1004-8. 225. Sadoshima, J.I., Izumo, S. Molecular character ization of angiotensin II induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Circ Res 1993, 73: 413-23. MEDICAMENTOS DE ACTUALIDAD 226. Schorb, W., Singer, H.A., Baker, K.M. Angioten sin II is a potent stimulator of MAP-kinase activ ity in neonatal rat cardiac fibroblasts. FASEB J 1993, 7(3): A492 (Abst). 227. Brilla, C.G., Matsubara, L., Weber, K.T. Inhibi tion of collagenase activity in cultured cardiac fibroblasts by angiotensin II. Eur Heart J 1992, 13(Suppl.): 111 (Abst). 228. Smits, J.F.M., VanKrimpen, C., Schoemaker, R.G., Cleutjens, J.P.M., Daemen, M.J.A.P. Angiotensin II receptor blockade after myocardial infarction in rats: Effects on hemodynamics, myocardial DNA synthesis, and interstitial colla gen content. J Cardiovasc Pharmacol 1992, 20: 772-8. 229. Schieffer, B., Wirger, A., Meybrunn, M., Setiz, S., Holtz, J., Riede, U.N., Drexler, H. Compara tive effects of chronic angiotensin converting enzyme inhibition and angiotensin H type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation 1994, 89(5): 2273-82. 230. Neuss, M., Regitz-Zagrosek, V., Hildebrandt, A., Fleck, E. Human cardiac fibroblasts express an angiotensin receptor with unusual binding characteristics which is coupled to cellular proliferation. Biochem Biophys Res Commun 1994, 204(3): 1334-9. 231. Smith, H.J., Nuttall, A. Experimental models of heart failure. Cardio Res 1985, 19: 181-6. 232. Matsumori, A., Tanaka, A., Wang, W., Sasayama, S. Angiotensin II receptor antagonist, TCV-116 improves virus induced myocardial injury in an animal model. Amer Heart Assoc 1994,90(4, Pt. 2): 1452 (Abst). 233. Trippodo, N.C., Panchal, B.C., Fox, M. Repres sion of angiotensin II and potentiation of bradykinin contribute to the synergistic effects of dual metalloprotease inhibition in heart failure. J Pharmacol Exp Ther 1995, 272(2): 619-27. 234. Ruzicka, M., Yuan, B., Leenen, F.H.H. Effects of enalapril versus losartan on regression of vol ume overload induced cardiac hypertrophy in rats. Circulation 1994, 90: 484-91. 235. Ruzicka, M., Keeley, F.W., Leenen, F.H.H. The renin angiotensin system and volume overload induced changes in cardiac collagen and elastin. Circulation 1994, 89(4): 1989-96. 236. Nishikimi, T., Ohmura, T., Tani, T., Yamagishi, H., Yanagi, S., Yoshiyama, M., Toda, I., Akioka, K., Teragaki, M., Takeuchi, K., Takeda, T. Role of angiotensin II type I receptor on the develop ment of heart failure in rats. J Amer Coll Cardiol 1993, 21(2): 27A (Abst). 237. Jiang, G.W., Mulroney, S.E., Opgenorth, T., Hoffman, A., Winaver, J., Haramati, A. Effects of DRUGS OF TODAY Vol. 31, No. 7, 1995 an angiotensin II receptor antagonist on urinary sodium excretion and cardiac hypertrophy in rats with experimental congestive heart failure. Xllth Cong Nephrol 1993, 242 (Abst). 238. Fujimori, A., Shibasaki, M., Kusayama, T., Okazaki, T., Matsuda, Y, Inagaki, O., Yanagisawa, I., Sato, N., Takenaka, T. YM358, a novel angio tensin II receptor (AT1) antagonist, prevents vol ume overload induced cardiac hypertrophy in rats. Can J Physiol Pharmacol 1994, 72(Suppl. 1): 120 (Abst). 239. Qing, G., Garcia, R. Chronic captopril and losartan (DuP 753) administration in rats with high output heart failure. Amer J Physiol 1992, 263(3): 833-40H. 240. Fitzpatrick, M.A., Rademaker, M.T., Charles, C.J., Yandle, T.G., Espiner, E.A., Ikram, H. Angiotensin II receptor antagonism in ovine heart failure: Acute hemodynamic, hormonal, and renal effects. Amer J Physiol 1992, 263: H250-6. 241. Murakami, M., Suzuki, H., Naitoh, M., Ichihara, A., Matsumoto, A., Tsujimoto, G., Saruta, T. Cardioprotective action of ACE inhibitor in failing heart. J Hypertens 1992, 10(Suppl. 4): S74 (Abst). 242. Ito, K., Kitayoshi, T., Igata, H., Goto, Y, Kito, G. Effect of TCV-116,a novel nonpeptide angioten sin II receptor antagonist, on chronic congestive heart failure. Can J Physiol Pharmacol 1994, 72(Suppl. 1): 120 (Abst). 243. Garcia-Sainz, J.A., Olivares-Reyes, J.A. Glycyl-histidine-lysine interacts with the angioten sin II AT1 receptor. Unpublished 1995. 244.Barbe, F., Jinbo, S., Guyenne, T.T., Laplace, M., Menard, J., Crozatier, B., Hittinger, L. The hypotensive effect of the angiotensin II antagonist EXP3174 depends upon the renin-angiotensin system activation in conscious dogs with heart failure. J Amer Coll Cardiol 1995, 371A (Abst). 245. Spinale, F.G., Hird, R.B., Mukherjee, R., Walker, J.D., Holzgrefe, H.H., Child, M.J., Koster, W.H. Chronic angiotensin II receptor block ade (AT1AT-II) affects myocyte sarcolemmal function and electrophysiology in dilated cardiomyopathy. J Amer Coll Cardiol 1995, 235A (Abst). 246. Smits, J.F.M., Schoemaker, R.G., Daemen, M.J.A.P., Debets, J.J.M. Haemodynamic conse quences of interference with the renin-angioten sin system following myocardial infarction in rats. Brit J Pharmacol 1991, 102(Suppl.): 9.9P (Abst). 247. Deck, C.C., Gaballa, M.A., Raya, T.E. Renal function in rats with experimental heart failure: Angiotensin II blockade versus converting 495 enzyme inhibition. Circulation 1993, 88(4, Pt. 2, Suppl.): 1-514 (Abst). 248. Stauss, H.M., Zhu, Y.C.; Redlich, T., Adamiak, D., Mott, A., Kregel, K.C., Unger, T. Angiotensin converting enzyme inhibition in infarct induced heart failure in rats: Bradykinin versus angioten sin II. J Cardiovasc Risk 1994, 1: 255-62. 249. Milavetz, J.J., Raya, T.E., Morkin, E., Goldman, S, Survival after large myocardial infarction in rats: Comparison of ACE inhibition with capto pril versus direct angiotensin II blockade with losartan. J Amer Coll Cardiol 1995,221A (Abst). 250. Kambayashi, Y, Takahashi, K., Bardhan, S., Inagami, T. Molecular structure and function of angiotensin type 2 receptor. Kidney Int 1994, 46(6): 1502-4. 251. DeGasparo, M., Bottari, S.P., Levens, N.R. Characteristics of angiotensin II receptors and their role in cell and organ physiology. In: Hyper tension: Pathophysiology, Diagnosis, and Man agement, 2nd Ed. Laragh, J.H., Brenner, B.M. (Eds.). Raven Press, Ltd., New York 1995, 1695-720. 252. Stadler, T., Veltmar, A., Qadri, F., Unger, T. Angiotensin II evokes noradrenaline release from the paraventricular nucleus in conscious rats. Brain Res 1992, 569(1): 117-22. 253. Simpson, R.L., Morlin, C., Ton, J., Snavely, D.B., Nelson, E.B., Goldberg, A.I. Efficacy and safety of losartan combined with hydrochlorothiazide in patients with mild to severe hyperten sion. Amer J Hypertens 1994, 7(4): A17 (Abst). 254. Arcuri, K., Harm, S., Nelson, E.B., Snapinn, S. Efficacy and safety of concomitant losartan/ hydrochlorothiazide therapy in patients with essential hypertension. Amer J Hypertens 1994,7(4): I48 (Abst). 255. Christen, Y, Waeber, B., Nussberger, J., Lee, R.J., Timmermans, P.B.M.W.M., Brunner, H.R. Dose-response relationships following oral administration of DuP 753 to normal humans. Amer J Hypertens 1991, 4(4, Pt. 2): 350-3S. 256. Munafo, A., Christen, Y, Nussberger, J., Shum, L., Borland, R.M., Lee, R J., Waeber, B., Biollaz, J., Brunner, H.R. Drug concentration response relationships in normal volunteers after oral administration of losartan, an angiotensin II receptor antagonist. Clin Pharmacol Ther 1992, 51:513-21. 257. Ohtawa, M., Takayama, F., Saitoh, K., Yoshinaga, T, Nakashima, M. Pharmacokinetics and biochemical efficacy after single and multiple oral administration of losartan, an orally active nonpeptide angiotensin II receptor antagonist, in humans. Brit J Clin Pharmacol 1993, 35: 290-7. 496 258. Goldberg, M.R., Tanaka, W., Barchowsky, A., Bradstreet, T.E., McCrea, J., Lo, M.W., McWilliams, E.J., Bjornsson, T.D. Effects of losartan on blood pressure, plasma renin activity, and angiotensin II in volunteers. Hypertension 1993, 21 (5): 704-13. 259.Burnier, M., Rutschmann, B., Nussberger, J., Versaggi, J., Shahinfar, S., Waeber, B., Brunner, H.R. Salt dependent renal effects of an angio tensin II antagonist in healthy subjects. Hyper tension 1993, 22(3): 339-47. 260. Cockcroft, J.R., Sciberras, D.G., Goldberg, M.R., Ritter, J.M. Comparison of angiotensin converting enzyme inhibition with angiotensin II receptor antagonism in the human forearm. J Cardiovasc Pharmacol 1993, 22(4): 579-84. 261. Goldberg, M.R., Bradstreet, T.E., McWilliams, E.J. et al. Biochemical effects of losartan, a nonpeptide angiotensin II receptor antagonist, on the renin angiotensin aldosterone system in hypertensive patients. Hypertension 1995, 25: 37-46. 262. Erley, C.M., Bader, B., Scheu, M., Wolf, S., Braun, N., Risler, T. Renal hemodynamics in essential hypertensives treated with losartan. Clin Nephrol 1995, 43(S1): S8-11. 263. Gradman, A.M., Arcuri, K., Goldberg, A.I., Ikeda, L.S., Nelson, E.B., Snavely, D.B., Sweet, C.S. A randomized, placebo controlled, double blind, parallel study of various doses of losartan potassium compared with enalapril maleate in patients with essential hypertension. Hyperten sion 1995, 25(6): 1345-50. 264.Weber, M.A., Byyny, R.L., Pratt, J.H., Faison, E.P., Snavely, D.B., Goldberg, A.I., Nelson, E.B. Blood pressure effects of the angiotensin II receptor blocker, losartan. Arch Intern Med 1995, 155(4): 405-11. 265. Nelson, E., Merrill, D., Sweet, C.S. et al. Effi cacy and safety of oral MK-954 (DuP 753), an angiotensin receptor antagonist, in essential hypertension. J Hypertens 1991, 9(Suppl. 6): S468-S469 (Abst). 266. Hagino, T., Abe, K., Tsunoda, K., Yoshinaga, K. Antihypertensive effect of a nonpeptide angio tensin II receptor antagonist, MK954, in patients with essential hypertension. Jpn J Nephrol 1992,34(2): 133-40. 267. Weber, M.A. Clinical experience with the angio tensin II receptor antagonist losartan. A prelimi nary report. Amer J Hypertens 1992, 5(12, Pt. 2): 247-51S. 268. Brunner, H.R., Christen, Y, Munafo, A., Lee, R.J., Waeber, B., Nussberger, J. Clinical experi ence with angiotensin II receptor antagonists. Amer J Hypertens 1992, 5(12, Pt. 2): 243-6S. MEDICAMENTOS DE ACTUALIDAD 269. Tsunoda, K., Abe, K., Hagino, T., Omata, K., Misawa, S., Imai, Y, Yoshinaga, K. Hypotensive effect of losartan, a nonpeptide angiotensin II receptor antagonist, in essential hypertension. Amer J Hypertens 1993, 6(1): 28-32. 270. Soffer, B.A., Wright, J.T., Pratt, J.H., Wiens, B., Goldberg, A.I., Sweet, C.S. Effects of losartan on a background of hydrochlorothiazide in patients with hypertension. Hypertension 1995, 26(1): 112-7. 271. Dahlof, B., Keller, S.E., Makris, L, Goldberg, A.I., Sweet, C.S., Lim, N.Y Efficacy and tolerability of losartan potassium and atenolol in patients with mild-to-moderate essential hyper tension. Amer J Hypertens 1995, 8(6): 578-83. 272. Shultz, P., Raij, L., Cook, M.E., Pearce, C., Buonagura, A., Wallin, J., Bauer, J. Effects of losar tan on renal function in patients with chronic renal insufficiency. Amer Soc Nephrol 1993,540 (Abst). 273. Gansevoort, R.T., DeZeeuw, D., DeJong, P.E. Is the antiproteinuric effect of ACE inhibition mediated by interference in the renin angioten sin system? Kidney int 1994, 45(3): 861-7. 274. Moan, A., Risanger, T., Eide, I., Kjeldsen, S.E. The effect of angiotensin II receptor blockade on insulin sensitivity and sympathetic nervous sys tem activity in primary hypertension. Blood Pressure 1994, 3(3): 185-8. 275. Lacourciere, Y, Lefebvre, J., Nakhle, G., Fai son, E.P., Snavely, D.B., Nelson, E.B. Associa tion between cough and angiotensin converting enzyme inhibitors versus angiotensin II antago nists: The design of a prospective, controlled study. J Hypertens 1994, 12(2): S49-53. 276. Lacourciere, Y, Brunner, H., Irwin, R., Karlberg, B.E., Ramsay, L.E., Snavely, D.B., Dobbins, T.W., Faison, E.P., Nelson, E.B. Effects of modu lators of the renin-angiotensin-aldosterone system on cough. J Hypertens 1994, 12(12): 1387-93. 277. Hayashi, H. Effect of MK-954 (losartan potas sium) on the circadian variation of ambulatory blood pressure - Investigation by the periodic analysis of covariance. Ther Res 1994, 15(12): 479-90. 278. Sweet, C.S., Bradstreet, D.C., Berman, R., Jallad, N.S., Saenz, A., Weidler, D.J. Pharmacodynamic activity of intravenous E 3174, an angio tensin II antagonist, in patients with essential hypertension. Amer J Hypertens 1994, 7(12): 1035-40. 279. Gottlieb, S.S., Dickstein, K., Fleck, E., Kostis, J., Levine, T.B., LeJemtel, T., DeKock, M. Hemodynamic and neurohormonal effects of the angiotensin II antagonist losartan in patients 497 with congestive heart failure. Circulation 1993, 88(4):1602-9. 280. Dickstein, K., Gottlieb, S., Fleck, E., Kostis, J., Levine, B., DeKock, M., LeJemtel, T. Hemodynamic and neurohumoral effects of the angiotensin II antagonist losartan in patients with heart failure. J Hypertens 1994, 12(2): S31-5. 281.Crozier, I., Ikram, H., Awan, N., Cleland, J., Ste phen, N., Dickstein, K., Frey, M., Young, J., Klinger, G., Makris, L., Rucinska, E. Losartan in heart failure: Hemodynamic effects and tolerability. Circulation 1995, 91: 691-7. 282. Dickstein, K., Chang, P., Willenheimer, R., Haunso, S., Remes, J., Hall, C., Kjekshus, J. Comparison of the effects of losartan and enalapril on clinical status and exercise performance in patients with moderate or severe chronic heart failure. J Amer Coll Cardiol 1995, 26(2): 438-45. 283. Ogihara, T., Mikami, H., Higaki, J. et al. TCV 116: Pharmacodynamics. Rinsho lyaku 1993, 9(5): 1031-63. 284. Ogihara, T., Higashimori, K., Masuo, K., Mikami, H. Pilot study of a new angiotensin II receptor antagonist, TCV-116: Effects of a single dose on blood pressure in patients with essential hypertension. Clin Ther 1993, 15: 684-91. 285. Strodter, D., Zeissig, I., Heath, R., Federlin, K. Angiotensin II antagonist CGP 48933 (valsartan) - Results of a double-blind, placebo con trolled multicenter study. Nier Hochdruckkrank 1994, 23(5): 217-20. 286. Muller, P., Cohen, T., DeGasparo, M., Sioufi, A., Racine-Poon, A., Howald, H. Angiotensin II receptor blockade with single doses of valsartan in healthy, normotensive subjects. Eur J Clin Pharmacol 1994, 47(3): 231-45. 287. Sissmann, J., Bouradian, M., Armagnac, C., Donazollo, Y., Latreille, M., Panis, R. Angioten sin II blockade in healthy volunteers: Tolerability and impact on renin angiotensin system compo nents of single and repeated doses of a new angiotensin II receptor antagonist SR 47436 (BMS 186295). J Hypertens 1994,12(Suppl. 3): Abst 510. 288. VanDenMeiracker, A., Janssen, J., Admiraal, P.J.J., deRonde, W., Kroodsma, J., Blankestijn, P.J., Sissmann, J. Dose finding study with angio tensin II receptor antagonist SR 47436 (BMS 186295) in essential hypertension. J Hypertens 1994, 12(Suppl. 3): 520 (Abst). 289. Burnier, M., Hagmann, M., Delacretaz, E., Brouard, R., Armagnac, C., Nussberger, J., Waeber, B., Brunner, H.R. The angiotensin II antagonist SR 47436 (BMS 186295) increases dose dependently urinary sodium excretion but not uric acid in healthy normotensive volunteers. J Hypertens 1994, 12(Suppl. 3): 545 (Abst). 290. Ribstein, J., Sissmann, J., Picard, A.Bouroudian, M., Mimran, A. Effects of the angiotensin II antagonist SR 47436 (BMS 186295) on the pressor response to exogenous angiotensin II and the renin angiotensin system in sodium replete normal subjects. J Hypertens 1994, 12(Suppl.3):2191 (Abst). 291. Hagmann, M., Burnier, M., Nussberger, J., Leenhardt, A.F., Brouard, R., Waeber, B., Brun ner, H.R. Natriuretic and hormonal effects of SR 47436 (BMS 186295), a new angiotensin II receptor antagonist in normotensive volunteers. Amer J Hypertens 1994, 7(4): 1370 (Abst). 292. Ribstein, J., Sissmann, J., Picard, A., Mimran, A. Prolonged blockade of the pressor response to exogenous angiotensin II by the angiotensin II antagonist SR 4 7436 (BMS 186295) in sodium replete normal subjects. Nephrol Dialysis Transplant 1994, 9(7): 928. 293. VanDenMeiracker, A.H., Admiraal, P.J.J., Janssen, J.A., Kroodsma, J.M., DeRonde, W.A.M., Boomsma, F, Sissmann, J., Blankestijn, P.J., Mulder, P.G.M., Man in't Veld, A.J., Schalekamp, M.A.D.H. Hemodynamic and biochemi cal effects of the AT 1 receptor antagonist irbesartan in hypertension. Hypertension 1995, 25: 22-9. 294. Burnier, M., Hagman, M., Nussberger, J., Biollaz, J., Armagnac, C., Brouard, R., Waeber, B., Brunner, H.R. Short term and sustained renal effects of angiotensin II receptor blockade in healthy subjects. Hypertension 1995, 25(4): P602-P609. 295. Su, C.A.P.F., VanHeiningen, P.N.M., Vanlier, J.J., de Bruin, H., Kirchgaessler, K.U., Cornelissen, P.J.G. Pharmacodynamics of the All antagonist BIBR0277SE. Clin Pharmacol Ther 1994, 55(2): 205 (Abst). 296. VanHeiningen, P.N.M., VanLier, J.J., deBruin, H., Su, C.A.P.F, Kirchgasessler, K.U., Cornelissen, P.J.G. Single dose study on the pharmacodynamics and pharmacokinetics of the angiotensin H antagonist BIBR0277SE. Pharm World Sci 1994, 16(3, Suppl. D): D4. 297. White, W.B., Mansoor, G.A., Lessem, J., Schusterman, N. Evaluation of the angiotensin, H receptor antagonist, eprosartan, by casual and ambulatory BP monitoring. Clin Res 1994, 42(3): 447A (Abst). 298. Naudin, R.B., Hagmann, M., Nussberger, J., Brunner, H.R. Oral administration of SC52458, a specific angiotensin II receptor antagonist, to 498 healthy male volunteers. J Hypertens 1994, 12(Suppl. 3): 517 (Abst). 299.Hagmann, M., Delacretaz, E., Nussberger, J., Naudin, R.B., Burns, T.S., Karim, A, Waeber, B., Brunner, H.R. Clinical and hormonal assess ment of SC-52458, a new orally active angiotensin II receptor antagonist in normotensive volun teers. Amer J Hypertens 1994, 7(4): B17 (Abst). 300.Buclin, T, Biollaz, J., Kung, S., Appenzeiler, M., Nussberger, J., Higgins, T., Obayashi, M., Brun ner, H.R. Tolerability, pharmacokinetics and pharmacodynamics of the angiotensin II (Ang II) antagonist TAK-536 in healthy volunteers. Clin Pharmacol Ther 1995, 57(2): P204. 301.Anonymous. Cozaar (losartan potassium tab lets). Merck & Co., Inc. 1995, 1-3. 302.Chiu, A.T., McCall, D.E., Price, W.A., Wong, P.C., Carini, D.J., Duncia, J.V., Wexier, R.R., Yoo, S.E., Johnson, A.L., Timmermans, P.B.M.W.M. In vitro pharmacology of DuP 753, a nonpeptide All receptor antagonist. Amer J Hypertens 1991, 4(4, Pt. 2): 282-7S. 303. Christ, D.D. Human plasma protein binding of the angiotensin If receptor antagonist losartan potassium (DuP 753/MK 954) and its pharma cologically active metabolite EXP3174. J Clin Pharmacol 1995, 35: 515-20. 304. Furtek, C.I., Lo, M.W.J. Simultaneous deter mination of a novel angiotensin II receptor block ing agent, losartan, and its metabolite in human plasma and urine by high-performance liquid chromatography. J Chromatogr Biomed Appl 1992, 573(2): 295-301. MEDICAMENTOS DE ACTUALIDAD 305. Hatch, M., Freel, R.W., Vaziri, N.D. Intestinal transport and renal excretion of urate is altered by a specific angiotensin II antagonist, DuP 753. Unpublished 1994. 306.Gansevoort, R.T., DeZeeuw, D., Shahinfar, S., Redfield, A., DeJong, P.E. Effects of the angio tensin II antagonist losartan in hypertensive patients with renal disease. J Hypertens 1994, 12(2): S37-42 (Abst). 307.Lang, R.M., Yeilen, L.G., McKelvie, R.S., Vaughan, D., Ney, D.E., Makris, L, Chang, P.I. Com parative effects of losartan and enal april on exercise capacity and clinical status in patients with heart failure. Circulation 1994, 90 (4, Pt. 2): I-602 (Abst). 308. Goldberg, A.l., Dunlay, M.C., Sweet, C.S. The safety and tolerability of losartan potassium, an angiotensin II receptor antagonist in compari son with hydrochlorothiazide, atenolol, felodipine ER and ACE-inhibitors for the treatment of systemic hypertension. Unpublished 1995. 309. Baboolal, K., Meyer, T.W. Acute angiotensin II receptor blockade does not reverse systemic or glomerular capillary hypertension in remnant kidney rats. J Amer Soc Nephrol 1992,3(3): 557 (Abst). 310. Timmermans, P.B.M.W.M., Carini, D.J., Chiu, A.T., Duncia, J.V., Price, W.A., Wells, G.J., Wong, P.C., Wexler, R.R., Johnson, A.L. The discovery of a new class of highly specific non peptide angiotensin II receptor antagonists. Amer J Hypertens 1991, 4(4, Pt. 2): 275-81S.