Schwartz et al study guide
advertisement

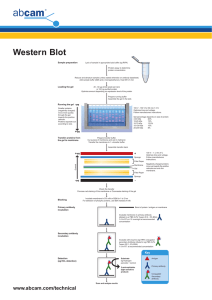
Schwartz et al study guide “modular domain” is a portion of a protein that has a certain property, and retains that property even in isolation (i.e. when separated from the rest of the protein it was originally found in—hence “modularity”) SH2 and SH3 domains are examples that we have discussed. Another is the FHA domain of Rad53 encountered in this paper. FHA domains bind a specific region of a protein that contains phosphorylated amino acids (e.g. Ser and Thr). PIKK = “phosphoinositide 3 kinase related kinase”; don’t worry about this name, just that it refers to a family of related kinases, of which ATM and ATR are members. MMS = methyl methane sulfonate, used to induce DNA damage SCD = S/T(Q) cluster domain, which is a place on the Rad9 protein that has a high density of amino acid residues potentially phosphorylated by ATM/ATR alanine substitution of Ser or Thr prevents phosphorylation Biochemical techniques used in the paper: Western or Immunoblot analysis: As we discussed several times, this is when a cell extract is made, the proteins are separated by size through a polyacrylamide gel, the proteins in the gel are transferred to a piece of nitrocellulose paper, and the nitorcellulose is incubated with antibodies that recognize a specific protein, which then shows up as a black “band”. This is not to be confused with IP/Western: In this technique a specific protein is immunoprecipitated from the cell extract using an antibody. This precipitate, which contains the protein you’re after (i.e. that the antibody recognized) and anything that sticks to it, is run through a gel, transferred, and then probed with an antibody. Sometimes this antibody is the same as the one you used for the precipitation-just to test that you precipitated your protein-, and sometimes it’s another antibody that recognizes some protein that you know or suspect will be co-precipitated by the first antibody. Rad53 phosphorylation can be detected by “mobility shift”; i.e. if it’s phosphorylated, then it runs slower through the gel and the bands are “shifted up” relative to the unphosphorylated protein BIOcore suface plasmon resonance: basically, if you see traces with resonance, then the proteins interact. They were measuring the interaction between the FHA domain of Rad53 and peptides containing T390 of Rad9. Kd = dissociation constant; the smaller the number the more tightly they bind cdc13 mutants are used as a tool to induce DNA damage genetically