Emulsion formation
advertisement

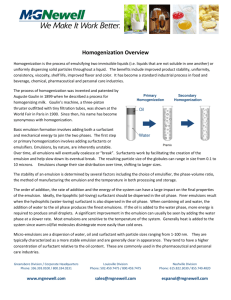
PHM2213 practical 106752546 PHM 2213 Physical Pharmacy 2 Preparation of oil-in-water emulsion NOTE Students will be divided into groups. Although experimental work will be carried out in a group, reports shall be submitted individually. OBJECTIVE This practical aims to allow students to understand the concept of emulsification by using different types of surfactants and the method to determine emulsion stability and properties and relate them to known theories. INTRODUCTION Emulsions have several advantages as drug delivery systems. For example, they offer opportunities for solubilising relatively large amounts of hydrophobic substances, with advantages relating to e.g. the effective drug solubility, the drug release rate, and the drug chemical stability, Furthermore, the amount of surfactants required are generally quite low, and relatively non-toxic surfactants such as phospholipids can be used as emulsifiers. Since oil and water do not mix, oil-water mixtures eventually separate into two macroscopic phases. Thus, emulsions are thermodynamically unstable systems. In order to form emulsions which are useful for drug delivery, their kinetic destabilisation must generally be slowed down by the use of different surface active agents. These may also help forming the emulsion and to reach a sufficiently small droplet size. The formation of emulsion systems is a complex process which involves generation and stabilisation of new oil-water interface. In drug delivery, high pressure homogenization is frequently used for generating new surface, and for producing emulsion droplets. Within high pressure homogenization, the liquid mixture is passed through a chamber where turbulent motions are generated. This, in turn, generates shear forces, which ‘tear apart’ any large entities in the system, and result in droplet formation. The size of the oil droplets formed depend on the intensity of emulsification i.e. the homogenization speed and period, temperature of the emulsification, ratio of the oil and water phases, type and dosage of the surfactants used and the geometry of the homogenization chamber. Indeed, the role of the surfactant is to reduce the interfacial tension and promote droplet formation and to stabilize the emulsion droplets from flocculation and coalescence. Prepared by: Kausar Ahmad Date created: 13-May-2004 Date of revision: 31-December-2004 Page 1 of 4 PHM2213 practical 106752546 EXPERIMENTAL PROCEDURE Note: The emulsions prepared in this practical will be used for subsequent practical sessions. Please keep the remaining emulsions at 30C. Label them: Course code Name of group leader Type of emulsion Date prepared 1. Preparation of phases for oil-in-water emulsion Weigh 200 g of palm oil/sunflower oil/canola oil/olive oil or otherwise given in a beaker. Weigh 600 g of distilled water in a one litre glass beaker. Ensure that the beakers are dry and free from contaminant. Prepare four emulsions: (i) without any surfactant (ii) non-ionic surfactant: 10% w/w (iii) anionic surfactant : 10% (iv) a combination of 5% non-ionic and 5% anionic surfactants Add the surfactants to either the oil phase or the water phase, depending on their compatibility. The amount of surfactants to be added is based on the amount of oil. Keep at 30C. Note To check the compatibility, try to dissolve a small amount of surfactant in oil and in water. Although not exclusive, emulsifiers can be selected based on Bancroft’s rule to produce either an oil-in-water emulsion or vice versa. Further, the hydrophilic-lipophilic balance (HLB) can be used as a guide to predict emulsion formation. 2. Homogenisation Immerse the homogenizer head into the water phase. Note the model of the homogenizer and record. Set the homogenizer speed at 4000 rpm or as allowed, depending on the equipment capability. Turn on the unit and add the oil phase. Turn the unit off after 10 minutes. Please confirm that the homogenizer head is completely immersed to avoid inclusion of air. 3. Phase separation Pour each emulsion formed into a 100 ml graduated cylinder. Keep at 30C. Observe the phase separation after 5 min, 10 min, 0.5, 1, 2 and 24 hours and record. Compare the extent of phase separation for the different emulsions produced. Prepared by: Kausar Ahmad Date created: 13-May-2004 Date of revision: 31-December-2004 Page 2 of 4 PHM2213 practical 106752546 4. Microscopy Take a picture of your emulsions immediately after preparation. One picture should contain at least 50 particles. Include the scale in the picture (this will allow you to calculate the size of your particles and compare to pictures that are to be taken later). RESULTS AND DISCUSSION Tabulate your results in the following manner: Table 1 Types of emulsions Emulsion No. Type of oil phase Oil concentration (% w/w) Surfactant type Surfactant name Surfactant concentration (% w/w) Table 2-1 Phase separation for emulsion no. 1 5 min 10 min 0.5 h Oil phase Cream phase Emulsion phase Water phase Remarks Table 2-2 Phase separation for emulsion no. 2 5 min 10 min 0.5 h Oil phase Cream phase Emulsion phase Water phase Remarks Table 2-3 Phase separation for emulsion no. 3 5 min 10 min 0.5 h Oil phase Cream phase Emulsion phase Water phase Remarks Table 2-4 Phase separation for emulsion no. 4 5 min 10 min 0.5 h Oil phase Cream phase Emulsion phase Water phase Remarks Prepared by: Kausar Ahmad Date created: 13-May-2004 Date of revision: 31-December-2004 Page 3 of 4 1h 2h 24 h 1h 2h 24 h 1h 2h 24 h 1h 2h 24 h PHM2213 practical 106752546 Write a report and submit one week after the practical. Plot your results based on Table 2-1 to 2-4 and answer the following in your discussion: 1. 2. 3. 4. What are the roles of surfactants? How does the surfactant impart stability? What is the effect of surfactant concentration on emulsion stability? What is the effect of homogenization conditions on the stability of the emulsions? a. emulsifying duration b. shear rate or homogenizing speed c. emulsifying temperature 5. From your results, what are the stabilisation and destabilization mechanisms involved for each emulsion? Explain. Prepared by: Kausar Ahmad Date created: 13-May-2004 Date of revision: 31-December-2004 Page 4 of 4