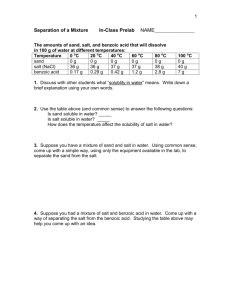
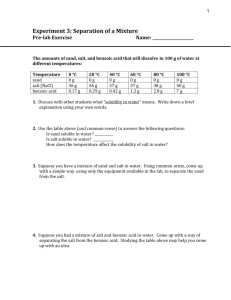
Name Date Period________ Separating a Mixture Lab Problem
advertisement

Separating a Mixture Lab Name ______________________________ Date _______________ Period________ Problem: How can a mixture be separated into its original parts? Hypothesis: If sand, salt, paper clips, and water are mixed in a beaker, then you can separate the paper clips through ___________________________, separate the sand through ______________________, separate the salt through _________________________ and separate the water through ______________________________. Materials: Procedure: hot plate salt (NaCl) measuring spoon aprons goggles 2 beakers 20 ml water (H20) graduated cylinder coffee filter beaker tongs sand (SiO2) paper clips rubber band metal scoopula bar magnet 1. 2. 3. 4. 5. 6. 7. 8. 9. Turn on hot plate. If it has a dial, turn to the highest setting. Place 15 mL of sand into the beaker. Place 15 mL of salt into the same beaker. Place 5 paper clips into the same beaker. Mix solid contents thoroughly. Add 20 mL of water into the same beaker. Mix the four items completely with the metal scoopula. Using the magnet, remove all paperclips from the mixture. On the second beaker, fasten the coffee filter to the beaker with a rubber band. Be careful not to tear the filter, and leave a little cushion (do not make it tight across). 10. Pour the mixture of sand, salt and water on the coffee filter. Allow this to drain completely. Carefully use the metal scoopula if necessary. 11. Remove the coffee filter from the beaker. 12. Place the beaker with the salt and water onto the hot plate and boil contents for approximately 5 minutes. 13. Observe the contents in the filter and the beaker. 14. Turn off and unplug your hotplate. 15. Throw away the filter and rinse out the beaker. Clean your station thoroughly. Data Questions: Answer in complete sentences on the back! 1. What physical process was used to separate the paper clips from the mixture? Explain. 2. What material was left in the filter? What is this process called? 3. What was left in the beaker after heating it? What is this process called? 4. What happened to the water in the beaker? 5. Was this a mixture or a compound? How do you know? 6. Was this an example of a heterogeneous or homogeneous mixture? How do you know? 7. The chemical composition of salt is NaCl (sodium chloride). Where you able to separate the sodium and chloride from each other? Why or why not?