6/26/14
X
DATE
REQUIRED COURSE
ELECTIVE COURSE
DIVISION
NEW COURSE
REVISION
x
LAKE LAND COLLEGE
Course Information Form
COURSE NUMBER
CHM 254
TITLE
SEM CR HRS 1.00
LT HRS
0.00
Organic Chemistry Laboratory 2
LAB HRS
3.00
SOE HRS
ECH
(Assigned by Administration)
COURSE PCS#
Prerequisites: CHM 243 and CHM 253
Catalog Description (40 Word Limit): Laboratory experiments in organic chemistry with a focus on
multi-step synthesis. Course is designed for concurrent registration in CHM 244.
List the Major Course Segments (Units)
Contact Lt Hrs
Contact Lab Hrs
Equipment and Safety Introduction
3
Vanillin Bromination
3
Nucleophilic Addition
3
Diels-Alder
3
Carbonyl Condensation
3
Natural Product Synthesis
15
Unexpected Synthetic Results
15
EVALUATION:
Textbook:
Quizzes
Lab Work
x
x
Exams
Projects
x
Oral Pres.
Comp Final
Title: Operational Organic Chemistry
Author: John W. Lehman
Publisher: Pearson / Prentice Hall
Volume/Edition:
4th
Copyright Date:
2009
x
Papers
Other
LAB CONTENT
Listed below are the experiments completed in this course.
Students are required to maintain a laboratory notebook in which they must include:
1. Prelab assignments including quantities of reactants required, the theoretical
yield of product, reactions, mechanisms, and procedures.
2. Observations Raw Data and Calculations
3. Results
4. Conclusions related to the problem and an explanation of how one arrived at these conclusions.
EXPERIMENTS: Some of these experiments require more than one three hour lab
period and are continued the following week.
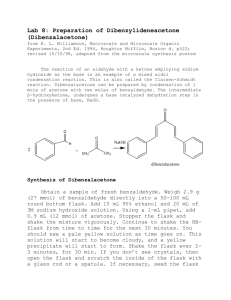
1. Bromination of Vanillin. Students a SEAr reaction on vanillin to illustrate the concept of directing
effects. The product is fully characterized by melting point, NMR, and IR. The students are
responsible for indicating how this data confirms the final location of the bromine atom.
Equipment used: electronic balance, Erlenmeyer flask, graduated cylinder, magnetic stirrer,
Buchner funnel, vacuum flask, melting point apparatus, NMR and IR spectrometers.
2. Wittig Reaction. Students perform a Wittig on trans-cinnamaldehyde to illustrate an example of
nucleophilic addition. Stereochemistry of the resulting alkenes is determined by melting point.
NMR and IR spectral data confirm loss of carbonyl. Equipment used: electronic balance,
Erlenmeyer flask, graduated cylinder, separatory funnel, magnetic stirrer, rotary evaporator,
Buchner funnel, vacuum flask, melting point apparatus, NMR and IR spectrometers.
3. Diels-Alder Reaction. Students carry out a simple Diels-Alder reaction involving maleic
anhydride and an unknown diene. The goal of the experiment is to successfully isolate and
characterize the adduct. Using the characterization information, the student should be able to
identify the unknown diene starting material. Equipment used: round bottom flask, reflux
condenser, Buchner funnel, vacuum flask, graduated cylinder, electronic balance, melting point
apparatus, IR spectrometer.
4. Knoevenagel Condensation. A microwave assisted Knoevenagel Condensation is carried out.
Additional experience is gained using the microwave reactor. The lab reinforces the baic
concepts of carbonyl condensation reactions. Equipment used: pipets, graduated cylinder,
microwave reaction vessel, microwave reactor, beaker, Hirsch funnel, vacuum flask, melting point
apparatus, NMR?IR spectrometers.
5. Natural Product Synthesis. Each student (individually) is required to complete the synthesis of
benzilic acid. The synthesis is a three step process. All intermediates must be isolated and
characterized by proton NMR, carbon NMR, IR, and melting point. Students must keep a detailed
record of their work in a lab notebook. Four lab periods are allotted for completion of the
synthesis. Equipment used: electronic balances, Erlenmeyer flask, pH paper, pipets, vacuum
flask, Buchner funnel, round bottom flask, reflux condenser, heating mantle, variac, beaker, glass
funnel, meting point apparatus, NMR/IR spectrometers.
6. Synthetic “Problems”. Students carry out a four step synthetic sequence where the last step
produces an unexpected product. The sequence utilizes four basic reactions such as Diels-Alder,
acid derivative hydrolysis, esterification, and alkene bromination. Each intermediate and the final
product must be completely characterized. Students must then speculate as to how and why the
unexpected product formed. At the conclusion of the project, each student is required to submit a
type written report. The report is written in the style of a peer reviewed journal article; complete
with abstract, introduction, results and discussion, conclusion, and experimental sections. Five to
six lab periods will be allotted for completion of the synthesis. Equipment used: fractional
distillation set up, Elrenmeyer flask, graduated cylinder, vacuum flask, Buchner funnel, Hirsh
funnel, round bottom flask, reflux condenser, hotplate, separatory funnel, rotary evaporator,
addition funnel, magnetic stirrer, melting point apparatus, NMR/IR spectrometers.
Major Course Segment
Hours
Learning Outcomes
Student will be able to:
Equipment and Safety
3
Identify various types of equipment and glassware used in an
organic laboratory. Assemble and operate equipment for
carrying out experimental techniques. Understand lab safety
Vanillin Bromination
3
Experimentally verify the position of the bromine during the
bromination of a multi-substituted aromatic ring. Understand
directing effects in SEAr reactions.
Wittig Reaction
3
Identify the stereochemistry of a generated alkene using
melting point. Understand nucleophilic addition.
Diels-Alder
3
Identify the structure of an unknown starting material through
the analysis of the reaction product.
Knoevenagel Condensation
3
Utilize a microwave reactor to successfully carry out a
carbonyl condensation reaction. Gain an appreciation for the
more environmentally friendly technique of
microwavechemistry.
Benzilic Acid Synthesis
15
Carry out and manage a multi-step synthetic sequence.
Prove the existence of each intermediate and of the final
product.
Unexpected Chemistry
15
Propose a synthetic scheme for a provided target. Carry out
the sequence, identifying each product along the way.
Recognize the formation of an unexpected product and
explain / rationalize its formation.
Course Outcomes: At the successful completion of this course, students will be able to:
Further master basic lab techniques such as reflux, distillation, recrystallization, extraction, and
separation of organic molecules.
Learn to keep a detailed “research style” lab notebook.
Learn how to report lab results to a group.
Gain experience in carrying out multi-step syntheses.
Develop scientific writing skills through the submission of a “publication style” paper.