Grignard_Reaction_Exper1
advertisement

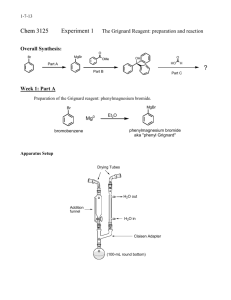
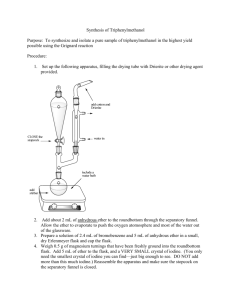
Glassware to be used in Grignard Reaction Your 250-mL round-bottomed flask, claisen connecting tube, condenser, 2 test tubes, and 125-mL separatory funnel, should be dry. Leave it out on your desktop for. PREPARATION OF TRIPHENYLMETHANOL H2SO4(aq) Eq: 2Mgo + 2C6H5Br + C6H5COCH3 ------------------> (C6H5)3· COH + CH3OH + 2Mg+2 + SO4-2 + 2Br Amounts of reactants actually used and maximum amounts possible of product Compound MW Mgo C6H5Br C6H5COOCH3 (C6H5)COH 24.0 157 136 260 1.522 1.094 Moles Grams 2.4 Density (g/ml) ml - 12.4 5.6 MPoC - -31 -12.4 164 BPoC - 156 196 - Assemble your apparatus as shown in the lab demo. 1. Working quickly, bring your reaction flask to the front of the room and add to it 2 shots (10 mL) of ANHYDROUS diethyl ether, and the contents of the vial of Mg provided. 2. Reassemble your reaction flask to your apparatus. 3. Take your separatory funnel (with stopcock CLOSED) to the front of the room and add to it 3 shots (9.3 mL) bromobenzene and 5 shots (25 mL) diethyl ether. Swirl the funnel to mix its contents and reassemble the funnel to your apparatus. 4. Prepare an ice bath in case it is needed. 5. Begin water running through your condenser. 6. Start your magnetic stirrer and add a 2- to 3-mL portion of the funnel contents to your reaction flask. 7. Take the dried test tube to the front of the room and add to it 1 shot of bromobenzene and 1 shot of ether. 8. Add enough magnesium turnings to barely cover the bottom of the tube. 9. With a stirring rod or spatula mix the contents of the tube. 10. Scrape the magnesium against the bottom and the sides of the tube frequently, to promote reaction between the bromobenzene and the magnesium. 11. Once the reaction has started, the ether will begin to reflux, and the tube will become warm to the touch, and subsequently the solution will turn brown. 12. At this point quickly remove your separatory funnel and add the contents of your tube to your flask. Return the separatory funnel to apparatus. When the contents of the flask begin to reflux, start adding the rest of the contents of your separatory funnel DROPWISE at a rate just fast enough to allow one drop of ether to fall from the condenser tip into this flask every second. Adding the contents too quickly causes coupling and overheating (see "explanations of procedure"). If the reaction becomes too vigorous, cool the reaction flask with your ice bath and reduce the rate of addition from your separatory funnel. If the reflux becomes too slow heat the reaction flask gently by cupping you’re your hands around the flask. The entire addition should be finished in 30 minutes. 13. Allow the reaction to proceed until only a few slivers of Mgo remain (about 2 hrs.). The contents of the flask now should be milky brown. 14. Once the Grignard Reagent has cooled to room temperature, add 5.6 mL of Methyl Benzoate and 20 mL anhydrous diethyl ether to your dropping funnel. 15. Begin s l o w, dropwise addition of the contents of the dropping funnel to the reaction flask. As in the preparation of the Grignard Reagent, control the rate of reaction by adjusting the rate of addition from the dropping funnel, and occasional icing of the reaction flask, should it become necessary. When the reflux has stopped the mixture may be heated to reflux for another 30 minutes to finish the reaction. Cool the flask. 16. Your reaction flask should contain approx. 100 mL of a solid/liquid mixture. IF NOT, add additional diethyl ether to bring the volume to about 100 mL and stopper the flask. DAY 2 17. In a 250-mL Erlenmeyer flask add enough ice to cover the bottom of the flask, followed by 50 mL of 6M H2SO4 - swirl the ice-acid mixture. 18, Add the contents of the reaction flask to the erlenmeyer, with swirling. Continue swirling until all solid matter has dissolved, and the solution is homogeneous. 19. Transfer the mixture back into the reaction flask, to collect any solid left behind. 20. Transfer this solution to a 250-mL separatory funnel and shake it vigorously but carefully, with frequent venting. If solid persists, add aliquots of ether. 21. Remove the aqueous layer from the funnel and pour the organic layer back into your reaction flask 22 Return the organic layer to your separatory funnel and wash the organic layer with 10 mL of 3M H2SO4. 23. Remove the aqueous layer from the funnel and wash the organic layer with 10 mL of saturated NaCl. 24. Repeat step #20 until the organic layer no longer tests acidic to blue litmus paper. 25. Dry the organic layer with anhydrous Na2SO4, and decant it into a 125 mL Erlenmeyer flask. 26. Boil off the ether on your hot plate set at "2”. The Triphenylmethanol remains in the flask. Pour your aqueous phases down the drain. 27. Recrystallize the residue from your flask, using a 2:1 mixture of cyclohexane:Absolute Ethanol; as follows: a. Place 40 mL of the solvent mixture into a 100 mL beaker. b. Place the beaker with solvent onto a hotplate and allow it to come to a boil. DO NOT ADJUST THE HEAT SETTING ON THE HOT PLATE - LEAVE IT AT "2" c. Remove the beaker of boiling solvent off the hot plate and add just enough solvent to dissolve the contents of your flask (swirl the flask contents during solvent addition, to insure only a minimum of solvent is used to dissolve your crude product.). The remainder of the solvent is to be iced, to wash your crystals. d. Transfer the contents of your flask to a 100 mL beaker. Allow your product to cool to room temperature on your desktop. e. f. Place the beaker in an ice bowl, to recrystallize your product. Vacuum filter the contents of the beaker, to recover your product. g. Wash your product with a small portion of ice-cold solvent. h. Leave the vacuum on for an additional 5 minutes, to help dry your product. i. Detach the hose from the filter flask then turn off the vacuum and place your product into your drawer to dry overnight. 28. Weigh your product and determine its melting range 29. Place your product in the jar provided in Hood at the end of the room. Laboratory Report. Follow outline given on page 5. Explanation of Procedure: Explanation of Procedure Steps 1-17 involve the equations of the reactions involved in the synthesis of triphenylmethanol. they are a. ether 2Mgo + 2 C6H5-Br ------> 2 C6H5-MgBr [oxidation] b. ether OMgBr C6H5-MgBr + C6H5-COOCH3 ------> C6H5 - C - C6H5 [addition] OCH3 c. OMgBr ether O C6H5-C-C6H5 -------> C6H5 - C - C6H5 + CH3OMgBr [elimination] OCH3 d. O ether OMgBr C6H5- C -C6H5 + C6H5-MgBr --------> C6H5 - C - C6H5 [addition] C6H5 e. OMgBr OH white gum CH3OMgBr + C6H5-C-C6H5 + H2O ---> C6H5-C-C6H5 + CH3OH + 2HOMgBr C6H5 C6H5 [hydrolysis] f. 2HOMgBr + H2SO4 -----> Mg+2 + SO4-2 + Br- + H2O [hydrolysis] g. 2Mgo H+(aq) + 2C6H5-Br + C6H5-COOCH3-------> 2Mg+2 + SO4-2 + Br- + CH3OH + OH C6H5-C-C6H5 C6H5 e. & f. are usually combined and written as OMgBr H2SO4(aq) OH C6H5-C-C6H5 + CH3OMgBr --------> C6H5-C-C6H5 + 2Mg + SO4-2 + BrC6H5 C6H5 because the H2SO4 is in water. Steps 1 & 2 The mechanism of the involvement of ether in the reactions is unknown but the following is postulated; for reaction a. the two molecules of ether may complex with Mg o to form CH3CH2OCH2CH3 Mgo CH3CH2COCH2CH3 Complex. If moisture is present, reactions a. to d. can be stopped by any of these side reactions: h. Mgo + 2H2O -----> Mg(OH) + H2 (gas) i. C6H5-MgBr + H2O ----> C6H5 + HOMgBr If much O2 is present, reaction j. can occur. j. 2C6H5-MgBr + O2 ----> 2C6H5-OMgBr and C6H5-OMgBr + H2O ---> C6H5-OH + HOMgBr if moisture is also present. If large amounts of CO2 are present ether 2C6H5-MgBr + 2CO2 --------> 2C6H5-COOMgBr, and when water is added Step 17 then 2C6H5-COOH + 2Mg + SO4 + 2Br Step 12 If the reaction (b.) becomes too vigorous and hot coupling can occur, which is C6H5-MgBr + C6H5-Br ----> C6H5-C6H5 + MgBr2 biphenyl In addition, the ether may evaporate stopping the reaction, undoing the complex ether with C6H5MgBr. Step 13-16 The addition of methyl benzoate is also exothermic and if it gets too hot the ether may evaporate, undoing the C6H5-MgBr-ether complex. Step 17-18 Involves reactions (e) and (f). the addition of acid along with the water (aqueous acid) prevents the white gum from forming. Some H 2 gas may be formed from reaction of the acid with Mgo [similar to reaction (h)]. Both methanol and triphenylmethanol are produced in these steps. Step 19. Look at the overall reaction (g) and notice that all your ions and probably much of the methanol will enter the aqueous phase. The triphenylmethanol and any small amounts of biphenyl, benzene, and benzophenone which may have formed because of side reactions, will remain in the ether layer. The washing with aqueous H2SO4 removes the inorganic ions and methanol. Step 20-21 Keeps the triphenylmethanol in the ether (salting out) while removing the H 2SO4. Step 22 Na2SO4 + nH2O -----> Na2SO4 10H2O(s). The liquid is now free of water. Step 23. Removes ether and any impurities such as benzene or biphenyl or benzophenone and leaves triphenylmethanol behind as a solid. Step 24. The cyclohexane reduces the polarity of the ethanol so that the triphenylmethanol will crystallize out. Step 24(i) Remember to disconnect the vacuum hose from the filter flask first, then turn off the water, otherwise the water from the faucet may back up into the filter flask and if you want the filtrate for any thing, it will be adulterated by the water. Step 25 Weigh your product on filter paper, determine its melting range and melting point. Put these data in the results of your notebook and laboratory report. In calculating the limiting reactant, be sure you choose the correct reactant by paying attention to the overall reaction (g) and your table. For the rest of the laboratory report, follow the outline given on page 5 of the syllabus. HOMEWORK Grignard Reagent Preparation 1. What is the limiting reagent in the preparation of the Grignard reagent? Show how you arrived at this conclusion. 2. Why are ether solvents important to the success of the preparation of the Grignard reagent ? 3. Why must the reagents, solvents and apparatus used for the preparation of the Grignard reagent be dry? 4. Why is it necessary to have an ice-water bath available during the preparation of the Grignard reagent? 5. What signs should you look for in determining whether the reaction has been initiated? 6. Why should the bromobenzene not be added all at once to the reaction flask ? 7. Ethanol is often present in the technical grade of diethyl ether. If this grade rather than anhydrous were used, what effect, if any, would the ethanol have on the formation of the grignard reagent ? Explain. 8. Why is it unwise to allow the solution of the Grignard reagent to remain exposed to air for an unnecessary period of time even if it is protected from moisture by drying tubes? 9. Why is it unwise to begin addition of the solution of methyl benzoate to the Grignard reagent before the latter has cooled to room temperature ? Preparation of Triphenylmethanol 1. What is the limiting reagent in the preparation of triphenylmethanol from phenylmagnesium bromide and methyl benzoate ? Show how you arrived at this conclusion. Why do you think this reagent rather than the other was made limiting ? 2. Why must the ester and the diethyl ether in which it is dissolved be anhydrous ? 3. Why is the solution of ester added to the Grignard reagent in a dropwise fashion rather than all at once? 4. Why is saturated aqueous sodium chloride rather than just water used to remove residual sulfuric acid from the organic solution during the workup procedure ? 5. How is unchanged ester removed from the desired product by the workup procedure used ? 6. What happens to any Grignard reagent that remains in the reaction mixture after addition of the ester ? 7. Why should anhydrous rather than technical grade diethyl ether be used to prepare the solution of methyl benzoate that is added to the Grignard reagent ? 8. What is the solid that forms during the addition of the ester to the Grignard reagent? 9. Why does pressure develop when the separatory funnel containing aqueous sulfuric acid and the ethereal solution of organic products is shaken ? 10. Why is the recrystallizing cyclohexane-ethanol (2:1) used instead of either alone?