Exam Review 2013
advertisement
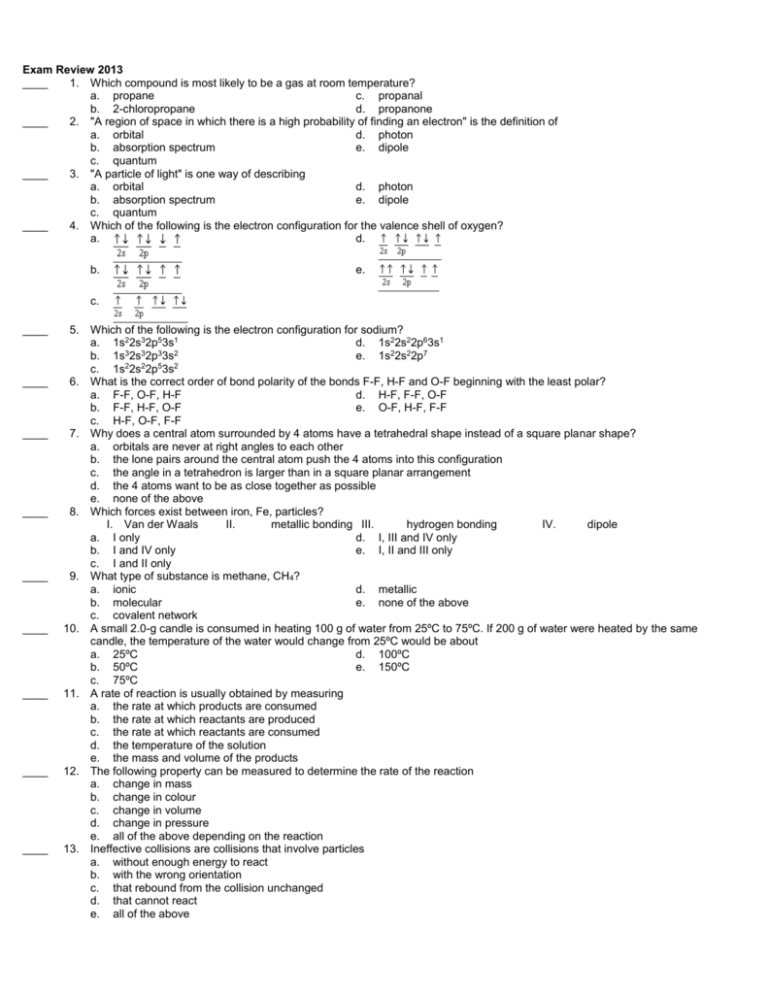
Exam Review 2013 ____ 1. Which compound is most likely to be a gas at room temperature? a. propane c. propanal b. 2-chloropropane d. propanone ____ 2. "A region of space in which there is a high probability of finding an electron" is the definition of a. orbital d. photon b. absorption spectrum e. dipole c. quantum ____ 3. "A particle of light" is one way of describing a. orbital d. photon b. absorption spectrum e. dipole c. quantum ____ 4. Which of the following is the electron configuration for the valence shell of oxygen? a. d. b. e. c. ____ 5. ____ 6. ____ 7. ____ 8. ____ 9. ____ 10. ____ 11. ____ 12. ____ 13. Which of the following is the electron configuration for sodium? a. 1s22s32p53s1 d. 1s22s22p63s1 b. 1s32s32p33s2 e. 1s22s22p7 2 2 5 2 c. 1s 2s 2p 3s What is the correct order of bond polarity of the bonds F-F, H-F and O-F beginning with the least polar? a. F-F, O-F, H-F d. H-F, F-F, O-F b. F-F, H-F, O-F e. O-F, H-F, F-F c. H-F, O-F, F-F Why does a central atom surrounded by 4 atoms have a tetrahedral shape instead of a square planar shape? a. orbitals are never at right angles to each other b. the lone pairs around the central atom push the 4 atoms into this configuration c. the angle in a tetrahedron is larger than in a square planar arrangement d. the 4 atoms want to be as close together as possible e. none of the above Which forces exist between iron, Fe, particles? I. Van der Waals II. metallic bonding III. hydrogen bonding IV. dipole a. I only d. I, III and IV only b. I and IV only e. I, II and III only c. I and II only What type of substance is methane, CH4? a. ionic d. metallic b. molecular e. none of the above c. covalent network A small 2.0-g candle is consumed in heating 100 g of water from 25ºC to 75ºC. If 200 g of water were heated by the same candle, the temperature of the water would change from 25ºC would be about a. 25ºC d. 100ºC b. 50ºC e. 150ºC c. 75ºC A rate of reaction is usually obtained by measuring a. the rate at which products are consumed b. the rate at which reactants are produced c. the rate at which reactants are consumed d. the temperature of the solution e. the mass and volume of the products The following property can be measured to determine the rate of the reaction a. change in mass b. change in colour c. change in volume d. change in pressure e. all of the above depending on the reaction Ineffective collisions are collisions that involve particles a. without enough energy to react b. with the wrong orientation c. that rebound from the collision unchanged d. that cannot react e. all of the above ____ ____ 14. Consider the above reaction mechanism. The rate-determining step of this reaction is a. elementary step 1 b. elementary step 2 c. elementary step 3 d. elementary steps 2 and 3 e. impossible to tell from this information 15. For the equilibrium system below, which of the following would result in a decrease in the quantity of PCl 5(g)? PCl3(g) + Cl2(g) <=====> PCl5(g) + 45 kJ a. increasing temperature d. decreasing the size of the container b. adding some Cl2(g) e. injecting some He gas c. decreasing temperature 16. For the equilibrium system below, which of the following would result in a decrease in the quantity of H 2(g)? N2(g) + 3H2(g) <=====> 2NH3(g) + 94 kJ a. decreasing the volume of the container d. removing some ammonia gas b. decreasing the temperature e. none of the above c. adding some ammonia gas 17. If the equilibrium constant for an equilibrium system is decreased by a decrease in temperature then a. [reactants] and [products] decreases b. [reactants] and [products] increases c. [reactants] increases and [products] decreases d. [reactants] decreases and [products] increases e. none of the above 18. A solution which conducts electricity is a(n) a. acid d. electrolyte b. base e. saturated c. salt 19. For sulfurous acid the Ka1 = a. [SO32-][H1+]2 / [H2SO3] d. [HSO31-][H1+] / [H2SO3] b. [HSO42-][H1+] / [H2SO3] e. [H2SO3] / [SO31-][H1+]2 11+ 2 c. [SO3 ][H ] / [H2SO3] 20. A solution of aluminum chloride has a pH of 6.126. The [OH1-] in mol/L must be which of the following? a. 7.874 d. 6.126 -7 b. e. none of the above -8 c. 21. A solution of potassium hydrogen sulfite has a pOH = 6.13. The [H 1+] is which of the following? -7 a. d. 7.87 -8 b. e. none of the above -8 c. -8, the Kb of its conjugate base partner must be which of the following? 22. -7 a. 6.20 d. -14 b. e. 7.80 -7 c. 23. Consider the following oxidation-reduction reaction: ____ Which of the following statements about this oxidation-reduction process is true? a. iodine is oxidized and potassium is reduced b. iodine is reduced and potassium is oxidized c. potassium is oxidized and chlorine is reduced d. chlorine is oxidized and hydrogen is reduced e. iodine is both oxidized and reduced 25. An electrochemical cell consists of a left compartment with a zinc electrode in contact with 1.0 mol/L ____ ____ ____ ____ ____ ____ ____ ____ right compartment with a silver electrode in contact with 1.0 mol/L and a . The standard reduction potentials are When this cell is allowed to operate at 25 C, which of the following statements is true? a. electrons will flow from right to left through the wire b. ions will be reduced to c. the concentration of ions in right compartment will increase the silver electrode will be the cathode the standard cell potential for this cell is 0.04 V d. e. ____ metal 26. Given for , which of the following are able to oxidize ? I. II. III. IV. ____ a. I and II d. I, III and IV b. II and III e. I, II and III c. I and IV 27. An electrochemical cell consists of a chromium electrode in 1.0 mol/L solution of electrode in 1.0 mol/L solution of (chromium nitrate), a silver (silver nitrate) and a salt bridge. The spontaneous cell reaction is When the two electrodes are connected by a wire, which of the following does not take place? a. the silver electrode increases in mass as the cell operates b. negative ions pass through the salt bridge from the silver half-cell to the chromium half-cell c. some positive chromium ions pass through the salt bridge from the chromium half-cell to the silver half-cell d. the chromium electrode decreases in mass as the cell operates e. electrons flow in the external circuit from the silver electrode to the chromium electrode ____ 28. The function of the salt bridge in a galvanic cell is a. to act as a nonelectrolyte b. to provide an external circuit for the flow of electrons from one half-cell to the other c. to provide a path for the migration of ions from one half-cell to the other d. to allow the solution in each half-cell to become electrically charged e. to provide a path for the flow of electrons internally from one half-cell to the other Short Answer 29. What would be the shape of NO3-1? Explain your reasoning. 30. Why do metals conduct electricity? 31. Differentiate between an effective collision and an ineffective collision. 32. How is a reaction mechanism designed? 33. If the pH of an acid solution at 25oC is 6.18, what is the pOH; and the [H1+], [OH1-] in mol/L? -4 mol/L, calculate the [OH1-] in mol/L, the pH, and the pOH. 34. If the [H1+] of a solution at 25oC is 1.7 35. Calculate for the following equation: Decide whether the equation represents a spontaneous or a nonspontaneous reaction at standard conditions. 36. What process occurs at the cathode of an electrolytic cell while the cell is operating? 37. Cubane is the name given to a newly synthesized alkane which has the formula C 8H8. This is a three-dimensional carboncarbon molecule where every carbon atom is bonded to three carbon atoms plus one hydrogen atom. Sketch the structure and explain why it tends to be unstable. 38. Write the reaction showing how the ester. , can be prepared from a carboxylic acid and an alcohol. Provide the names of all reactants below your equation. 39. The structure given below is a polymer classified as a polyamide. This polymer is stable in temperatures above 350°C, and can be used as a protective coating on hot surfaces. What monomers were used to make this polyamide? 40. 41. 42. 43. Explain why CH3F is a polar molecule while CF4 is not. Is ammonia a polar molecule? Explain your answer using a diagram. Predict the shape and bond angles of hydrogen sulphide, H2S. Explain why . 44. 45. 46. 47. 48. 49. 50. 51. 52. How does the rate of change of the product compare to the rate of change of the reactant? Describe how the chemical nature of the reactants can control the rate of the reaction. Give an example. How can the effect of concentration on the reaction rate be explained by collision theory? Consider the equilibrium below: If 1.2 mol of H2 and 1.2 mol of O2 was placed in a 1.0 L container and allowed to reach equilibrium, what would the value of Ke be if at equilibrium [HI] = 0.40 mol/L? H2(g) + I2(g) <=====> 2HI(g) Consider the equilibrium below: If 1.2 mol of HI was placed in a 1.0 L container and allowed to reach equilibrium what would the value of Ke be if at equilibrium [HI] = 0.40 mol/L. H2(g) + I2(g) <=====> 2HI(g) Consider the equilibrium below: If 3.4 mol of HI was placed in a 2.0 L container and allowed to reach equilibrium, what would the equilibrium concentrations be for H2(g), I2(g) and HI(g) if the Ke = 49? H2(g) + I2(g) <=====> 2HI(g) The Ke for the equilibrium shown below is 50. Some methane and hydrogen sulfide are added to a container and at equilibrium the concentrations of methane and hydrogen sulfide are found to be 0.24 mol/L and 0.36 mol/L respectively. What are the equilibrium concentrations of carbon disulfide and hydrogen gas? CH4(g) + 2H2S(g) <====> CS2(g) + 4H2(g) If the pOH of an acidic solution at 25oC is 12.69, what is the pH; and the [H1+], [OH1-] in mol/L? Write the Kb expression for the weak base NO2-1(aq). 53. State whether the change of ion to 54. What is the oxidation number of Cl in ion is an oxidation or a reduction. ? 55. Calculate the enthalpy change, for the vaporization of 200 g of methanol. o for the 56. Ethanol, C2H5OH, is made industrially by the reaction of water with ethylene, C2H4 reaction C2H4(g) + H2 2H5OH(l) given the following thermochemical equations: o = –1411.1 kJ C2H4(g) + 3 O2 2(g) + 2 H2 o = –1367.1 kJ C2H5OH(l) + 3 O2 2(g) + 3 H2 57. If one mole of water absorbs 44 kJ of heat as it changes state from liquid to gas, calculate the amount of heat that is absorbed if 200 g of water is evaporated. -09 mol/L, what is its Ksp? 58. If the solubility of Al(OH)3 -4 mol/L solution of KI, would a 59. If 365 mL of a 0.0054 mol/L solution of Pb(NO3)2 -9. precipitate form? Calculate the ion product for the potential precipitate. The Ksp of PbI 2 -11 and its pH = 8.75? 60. What is the concentration of a weak base if its Kb -6? 61. What is the percent ionization of a 1.38 mol/L weak acid if its Ka 62. A weak base with a concentration of 0.66 mol/L has a percent ionization of 1.4 %. What is the Kb of this weak base? 63. Farmers in the south often spray water on plants when there is a chance of frost to protect them from freezing. Calculate the amount of heat released when 100 kg of water freezes at 0ºC. 64. Consider the following equation for the combustion of H2(hydrogen): H2(g) + 1/2O2 2O(g) + 243 kJ In order to produce 972 kJ of heat, how many grams of H2 must burn? 65. a. Calculate Ho for the following reaction. b. State whether the reaction is exothermic or endothermic. c. Rewrite the equation as a thermochemical equation to include the heat term. d. Indicate whether the products have a greater or smaller enthalpy than the reactants. -11? 66. What is the pH of a 1.47 mol/L solution of HCN(aq) if its Ka = 3.5 67. What is the pH of a 3.4 mol/L solution of KOH? 68. Balance the following equation by the half-reaction method: + + + 69. Balance the following equation by the oxidation number method and identify the oxidizing and reducing agents: 70. Balance the following redox reaction which takes place in an acidic solution using the half-reaction method: 71. Balance the following redox reaction which takes place in a basic solution using the half-reaction method: Exam Review 2013 Answer Section MULTIPLE CHOICE 1. ANS: STA: A OC1.02 PTS: 1 REF: K/U OBJ: 1.1 2. ANS: STA: A SP2.01 PTS: 1 REF: C OBJ: 3.5 3. ANS: STA: D SP2.01 PTS: 1 REF: C OBJ: 3.3 4. ANS: STA: B SP2.02 PTS: 1 REF: C OBJ: 3.6 5. ANS: STA: D SP2.02 PTS: 1 REF: C OBJ: 3.6 6. ANS: STA: A SP2.04 PTS: 1 REF: I OBJ: 4.4 7. ANS: STA: C SP1.05 PTS: 1 REF: K/U OBJ: 4.3 8. ANS: STA: C SP1.04 PTS: 1 REF: K/U OBJ: 4.5 9. ANS: STA: B SP2.05 PTS: 1 REF: I OBJ: 4.6 10. ANS: STA: A EC2.03 PTS: 1 REF: K/U OBJ: 5.2 11. ANS: STA: C EC2.01 PTS: 1 REF: K/U OBJ: 6.1 12. ANS: STA: E EC2.01 PTS: 1 REF: K/U OBJ: 6.1 13. ANS: STA: E EC1.04 PTS: 1 REF: K/U OBJ: 6.4 14. ANS: STA: A EC1.06 PTS: 1 REF: K/U OBJ: 6.4 15. ANS: STA: A CS1.03 PTS: 1 REF: I OBJ: 7.3 16. ANS: STA: C CS2.06 PTS: 1 REF: I OBJ: 7.5 17. ANS: STA: C CS1.03 PTS: 1 REF: K/U OBJ: 7.3 18. ANS: STA: D CS1.06 PTS: 1 REF: K/U OBJ: 7.1 19. ANS: STA: D CS2.06 PTS: 1 REF: K/U OBJ: 8.2 20. ANS: STA: C CS2.06 PTS: 1 REF: I OBJ: 8.2 21. ANS: C PTS: 1 REF: I OBJ: 8.2 STA: CS2.06 22. ANS: STA: D CS2.06 PTS: 1 REF: I OBJ: 8.2 23. ANS: STA: E EL1.01 PTS: 1 REF: K/U OBJ: 9.1 24. ANS: STA: C EL2.02 PTS: 1 REF: C OBJ: 9.3 25. ANS: STA: D EL2.04 PTS: 1 REF: C OBJ: 9.5 26. ANS: STA: E EL2.05 PTS: 1 REF: C OBJ: 9.3 27. ANS: STA: E EL2.04 PTS: 1 REF: C OBJ: 9.5 28. ANS: STA: C EL1.02 PTS: 1 REF: K/U OBJ: 9.5 SHORT ANSWER 29. ANS: It would be a trigonal planar molecule, because nitrogen would have three oxygen atoms and no unbonded pairs of electrons around it. This configuration would get the bonded electrons as far apart as possible. PTS: 1 REF: I OBJ: 4.3 STA: SP2.03 30. ANS: The delocalized electrons are free to move from one place to another, thus forming an electric current. PTS: 1 REF: I OBJ: 4.6 STA: SP2.05 31. ANS: in an effective collision, the particles collide with sufficient energy and correct orientation for a reaction to occur, the formation of products occurs from this collision in an ineffective collision, the particles collide without sufficient energy and/or correct orientation for the reaction to occur, the reactants are unchanged by this collision PTS: 1 REF: K/U OBJ: 6.4 STA: EC1.04 32. ANS: each step must be elementary, involving not more than three reactant (and more usually only one or two) molecules the slowest or rate-determining step must be consistent with the rate equation the elementary steps must add up to the overall equation PTS: 1 REF: 33. ANS: pOH = 7.82, [H1+ PTS: PTS: OBJ: 10-7 mol/L., [OH1- 1 34. ANS: [OH1- K/U REF: -11 I 6.4 STA: EC1.06 8.2 STA: CS2.06 8.2 STA: CS2.06 -8 OBJ: mol/L. mol/L, pH = 3.77, pOH = 10.23 1 35. ANS: Reduction half-reaction: Oxidation half-reaction: REF: I OBJ: Overall reaction: for the overall reaction is positive, thus the reaction is spontaneous as written. PTS: 1 REF: C OBJ: 9.3 STA: EL2.05 36. ANS: Reduction occurs at the cathode of an electrolytic cell while the cell is operating. PTS: 1 REF: K/U OBJ: 10.1 STA: EL1.02 37. ANS: - exists as a cube angles between C-C bonds are only 90 Since bonding electrons are close together, they tend to repel, making this molecule unstable bond strain is high PTS: 1 REF: C OBJ: 1.2 STA: OC2.03 1 REF: C OBJ: 1.7 STA: OC2.05 OBJ: 2.2 STA: OC2.05 38. ANS: PTS: 39. ANS: is a polyamide polymer monomers are: , PTS: 1 REF: C 40. ANS: Both species have a tetrahedral structure. This shape is symmetrical in all directions. Therefore, even though the CF 4 molecule contains four polar bonds, there is no positive end and negative end; therefore the molecule itself is not polar. However, the CH3F molecule contains only one C-F bond. The F draws electrons from the rest of the molecule as well, forming a partial negative charge at its end, and leaving the hydrogens with partial positive bonds. PTS: 1 REF: I OBJ: 4.4 STA: SP2.04 41. ANS: Ammonia would be polar because it has a lone pair that pushes the hydrogens down, forming an asymmetric molecule. Also, nitrogen is highly electronegative, so each N-H bond is polar. PTS: 1 REF: I OBJ: 4.4 STA: SP2.04 OBJ: 4.3 STA: SP2.03 42. ANS: bent with bond angle of less than 109o PTS: 1 REF: I 43. ANS: Due to the Law of Conservation of Mass, the total amount of energy in the system must remain constant; consequently, the enthalpy change of the system must be equal in magnitude, but opposite in sign to the energy released to or absorbed from the surroundings. PTS: 1 REF: K/U OBJ: 5.1 STA: EC2.02 44. ANS: - rate of changes are opposite in sign, that is as the reactant decreases, the product increases - however, the magnitude of change may or may not be equal in magnitude. Depending on the ratio of the coefficients in the balanced reaction PTS: 1 REF: K/U OBJ: 6.1 STA: EC1.03 45. ANS: Although chemicals can be very similar in nature, be it acids, bases, metals, or gases, they all tend to react at different rates. (e.g., Alkali metals all react at a different rate - as you go down the group, the reactions occur faster; acids all have different strengths, Hydrochloric acid would react much faster than acetic acid.) PTS: 1 REF: C OBJ: 6.2 STA: EC1.04 46. ANS: if the initial concentration of the reactant is increased, the rate generally increases because a greater number of particles per unit volume which are more likely to collide if there are more collisions, the rate at which effective collision occur will increase causing an increase in the rate of the reaction PTS: 1 REF: K/U OBJ: 6.5 STA: EC1.04 7.5 STA: CS2.06 7.5 STA: CS2.06 47. ANS: mol/L H2(g) + I2(g) <=====> 1.2 1.2 initial shift –0.20 –0.20 @E 1.0 1.0 Ke = (0.40)2 / (1.0 PTS: 1 REF: I 2HI(g) 0.40 0.40 OBJ: 48. ANS: mol/L H2(g) + initial shift 0.40 @E 0.40 Ke = (0.40)2 PTS: 1 I2(g) <=====> 1.2 0.40 0.40 REF: I 2HI(g) –0.80 0.40 OBJ: 49. ANS: mol/L initial H2(g) + I2(g) <=====> 1.7 2HI(g) – shift @E 2 2) = 49 (take the square root of both sides) 2] = [I2 – PTS: 1 REF: 50. ANS: mol/L @E CH4(g) + 0.24 I 2H2S(g) <====> 0.36 OBJ: 7.5 CS2(g) + STA: CS2.06 STA: CS2.06 4H2(g) 4 5 5 / 0.0864 = (50)(0.0864)(256) 2]; [H2] = 16 mol/L PTS: 1 REF: 51. ANS: pH = 1.31, [H1+ PTS: 1 -2 I mol/L., [OH1- REF: OBJ: -13 7.5 mol/L. I OBJ: 8.2 STA: CS2.06 K/U OBJ: 8.2 STA: CS2.06 52. ANS: Kb = [HNO2][OH1-] / NO21PTS: 1 REF: 53. ANS: The change is a reduction. PTS: 1 REF: K/U OBJ: 9.1 STA: EL1.01 1 REF: K/U OBJ: 9.1 STA: EL1.01 54. ANS: +7 PTS: PROBLEM 55. ANS: Find the number of moles of methanol Then find the enthalpy change Since the methanol vaporizes by absorbing heat, the enthalpy change is +244.8 kJ. PTS: 1 REF: I OBJ: 5.2 STA: EC2.03 56. ANS: Show the addition of equations to give "target" reaction C2H4(g) + 3 O2(g) 2(g) o= + 2 H2O(l) –1411.1 kJ o = +1367.1 kJ 2 CO2(g) + 3 H2 2H5OH(l) + 3 O2(g) ________________________________________________________________ C2H4(g) + H2 The PTS: o= 2H5OH(l) –44 kJ Ho for the reaction is 44 kJ. 1 REF: I OBJ: 5.4 STA: EC2.04 STA: EC2.02 STA: CS2.06 57. ANS: Calculate the number of moles of water Calculate heat 4.9 x 102 kJ of heat would be absorbed in the evaporation. PTS: 1 REF: 58. ANS: Al(OH)3(s) <====> @E 1 OBJ: Al3+(aq) -09 3.2 -09 PTS: I 3 OH1-(aq) + -09 -09 )3 REF: 5.2 -33 I OBJ: 7.6 59. ANS: Pb(NO3)2 2(s) + 2KNO3(aq) [Pb2+] = (0.0054 mol/L)(0.365 L) / (0.365 L + 0.595) L -4 mol/L)(0.595 L) / (0.365 L + 0.595) L [I1ion product PbI2 is [Pb2+][I1-]2 PTS: 1 REF: -10 < Ksp, no precipitate forms I OBJ: 60. ANS: XOH(aq) <=====> X1+(aq) + OH1-(aq) x 10-pOH 10-pOH 7.6 STA: CS2.05 pOH = 14 – pH = 14 – 8.75 = 5.25 [OH1-] = 10-pOH = 10-5.25 -6 mol/L [OH1-6)2 -11 mol/L -6)2 / -11 = 2.3 mol/L PTS: 1 REF: I OBJ: 8.2 STA: CS2.06 61. ANS: HY(aq) <====> H1+(aq) + Y1-(aq) 1.38 -x initial shift @E x2 / x x x x -6 -6 0.5 pH = – log x pH = 2.71, % Ionization = 100(x / 1.38) = 0.14% PTS: 1 REF: I OBJ: 8.2 STA: CS2.06 62. ANS: X1+ XOH <=======> 0.66 initial shift @E 0.66 (0.66)(1.4)/100 Ka = ((0.66)(1.4)/100)2 PTS: 1 + OH1- (0.66)(1.4)/100 -4 REF: I OBJ: 8.2 STA: CS2.06 63. ANS: Calculate the number of moles of water: Calculate the enthalpy change 33.4 MJ of heat would be released (to protect the plants) when 100 kg of water freezes. PTS: 64. ANS: 1 REF: I OBJ: 5.2 STA: EC2.03 Determine the number of moles of H2 that are required. Calculate the mass of H2 required 8.08 g of H2 would need to be burned. PTS: 1 REF: I OBJ: 5.3 STA: EC2.03 65. ANS: a. The heat of reaction is –907 kJ/mol of Benzene b. Since the sign of the heat of reaction is negative, the reaction is exothermic. c. d. Since the reaction is exothermic, the products would have a smaller enthalpy than the reactants, since energy is given off to the surroundings. PTS: 1 REF: I OBJ: 5.5 STA: EC2.05 | EC2.02 8.2 STA: CS2.06 8.2 STA: CS2.06 66. ANS: HCN(aq) <====> H1+(aq) + CN1-(aq) 1.47 initial shift @E -x x2 x x x x -11 -11 0.5 pH = – log x pH = 4.56 pH = 5.14 PTS: 1 REF: I OBJ: 67. ANS: KOH is completely ionized since it is a strong acid. [OH1-] = 3.4 mol/L pOH = –log [OH1-] = –0.53 pH = 14 – pOH pH = 14.53 PTS: ESSAY 1 REF: I OBJ: 68. ANS: The unbalanced reduction half-cell reaction is: The unbalanced oxidation half-cell reaction is: The balanced reduction half-cell reaction is: + + + The balanced oxidation half-cell reaction is: Balance the number of electrons in the balanced two half-cell reactions above by multiplying the oxidation half-cell reaction by 5: + + 5 + 5 5 The net algebraic sum of the two half-cell reactions is the balanced equation for the reaction in question: + PTS: 1 REF: +5 C +5 OBJ: 9.2 + STA: EL2.03 69. ANS: -1 +6 2 +1 0 +1 2 +1 2 The change in the oxidation number of the oxidized element I = (0) – (–1) = +1 The change in the oxidation number of the reduced element S = (–2) – (+6) = –8 The lowest common multiple of 1 and 8 is 8. Thus in the balanced reaction eight iodine atoms are needed for every sulfur atom. The increase and decrease in oxidation numbers will then be eight for both. The partially balanced equation is: The remaining coefficients can be balanced by inspection and the balanced equation is: The oxidizing agent is: PTS: 1 . The reducing agent is REF: C OBJ: . 9.2 STA: EL2.03 70. ANS: The unbalanced reduction half-cell reaction is: The unbalanced oxidation half-cell reaction is: The balanced reduction half-cell reaction is: The balanced oxidation half-cell reaction is: Balance the number of electrons in the balanced two half-cell reactions above by multiplying the oxidation half-cell reaction by 6: The net algebraic sum of the two half-cell reactions is the balanced equation for the reaction in question: PTS: 1 REF: C 71. ANS: The unbalanced reduction half-cell reaction is: The unbalanced oxidation half-cell reaction is: OBJ: 9.2 STA: EL2.03 The balanced reduction half-cell reaction is: The balanced oxidation half-cell reaction is: Balance the number of electrons in the balanced two half-cell reactions above by multiplying the reduction half-reaction by 3 and the oxidation half-cell reaction by 8: The net algebraic sum of the two half-cell reactions is: Since the reaction occurs in a basic medium, we add equation for the reaction: PTS: 1 REF: C OBJ: to both sides and the net equation obtained is the balanced 9.2 STA: EL2.03







