MicroMole Rockets
advertisement

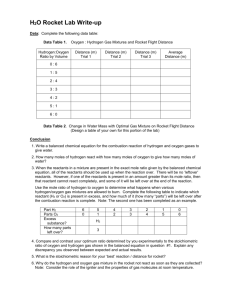
MicroMole Rockets A small plastic rocket is constructed from the calibrated bulb of a graduated plastic transfer pipet. This bulb is filled with water, and then most of the water is displaced - first with hydrogen and then oxygen. Electrodes are inserted into the device. A spark causes the mixture to ignite and launch the rocket. Students are challenged to find the most "powerful" mixture and then use it to launch a rocket across the room! Objectives Hydrogen (H2) and oxygen (O2) are two gases that react with each other in a very quick, exothermic (heat producing) manner. The explosiveness of this reaction is greatest when the hydrogen and oxygen are mixed in just the proper proportion. In this lab, you are generating hydrogen and oxygen and testing their explosive nature -- first separately, then in mixtures of different proportions. Your goal is to find the most "powerful" mixture and then use it to launch a rocket across the room! • Observe and Compare two gas-producing reactions. • Apply the concept of combining ratios. Chemical Concepts 1 Hydrogen reacts with oxygen in a very quick and exothermic manner. 2 Additional energy is often required to get reactant particles to collide hard enough to initiate a reaction; this extra energy is known as the activation energy. Electric sparks add energy to the molecules. 3 Gas volumes in this experiment are directly related to the moles present. The combining volumes can be used to determine the ratio of the combining moles of material. 4 The molar ratio of reactants consumed depends on the chemical equation. Either reagent in a reaction may limit the extent of the reaction. Safety • Safety goggles and apron must be worn throughout this activity. HCl is a strong corrosive acid. Wash all acid spills immediately with excess soap and water. Avoid ingesting the chemicals. The gas generators are more dangerous than the rockets. • Wear your goggles as self-defense. • Remember, others are using acid and shooting rockets too. • Use the assigned launch pad and target area. Procedure 1 Water Supply: Fill the petri dish 3/4 full with tap water. This serves as a recycling water supply during the experiment. 2 Practice filling the bulbs entirely with water: Squeeze the pipet, dip the mouth in the petri dish of water and release the squeeze to draw up some water. Then, with the bulb held mouth upward, squeeze a second time, just to the point where the water inside the pipet starts to come out; then, still squeezing, dip the mouth into the petri again and draw up the remaining water needed to fill the bulb. 3 Generating the Hydrogen: The H2 generator is a plastic vial containing several pieces of zinc metal (Zn) and capped with a nozzle-fitted lid. Remove the lid and carefully add enough 1.0 M HCl to fill the vial to within 1 cm of the top. Replace the lid and set the generator in the petri dish of water. Observe. 4 Generating the Oxygen: The O2 generator consists of an empty plastic vial capped with nozzlefitted lid. Remove the lid and add enough 3% H2O2 to fill the vial to within 1 cm of the top. Use10 drops of yeast water (the catalyst). Replace the lid, swirl gently, and set the generator in the petri dish beside the H2 generator. Observe. 5 Collecting a Sample of Hydrogen Gas by Water Displacement: Fill the collection bulb completely with water from the petri dish, and place it mouth-downward over the nozzle of the H2 generator. The fit should be loose, enabling water to leak out as the bulb collects the hydrogen gas. As soon as the bulb is completely filled with the hydrogen, remove it from the nozzle and place a finger over the opening to prevent the collected gas from leaking out. 6 Pop-test: Move your finger aside and insert the wire tip of the piezo sparker into the bulb. Holding the bulb securely, pull the trigger and observe. Record the loudness of the reaction (on a scale of 1-10 where 10 is loudest) in the first column of the data table. 7 Collecting and Pop-Testing Oxygen: Repeat steps 5 and 6, collecting oxygen gas this time, and pop-testing it with the piezo sparker. Record the loudness of the reaction (on a scale of 1-10) in the last column of the data table. 8 Collecting and Testing Different Mixtures: Begin to collect another bulb of O2, but after the bulb is one-sixth full, move the bulb from the O2 generator to the H2 generator and continue collecting; this gives you a 1:5 mixture of O2 and H2. Pop-test it and record its relative loudness in the appropriate box (second from the left) in the data table below. Then repeat this procedure, making the switch-over at various points so as to create mixtures of various proportions (2:4, 3:3, 4:2, and 5:1). Record relative loudness in the appropriate data table boxes. Note the amount of light also. Note: Should either of the two generator reactions slow down to a point where it takes longer than one minute to collect a bulb, simply lift off the collection bulb and set it down on the lab bench, remove the lid from the generator, decant off the remaining liquid into the appropriate waste container, replace the liquid with fresh solution (and catalyst for the oxygen generator) from the appropriate bottle, replace the lid and resume gas collection. 9 Rocket Launch! Once you have determined the optimum (loudest) mixture of the two gases, collect it again, and bring it, along with your piezo sparker to the launch site designated by the instructor. This time, instead of holding onto the bulb, aim it outward, at a designated target. Making sure nobody is in the line of fire, pull the trigger and launch your micro rocket. Can you think of ways to make your rocket go farther? Try them! Name Date Period Micromole Rockets: Pre-lab questions 1. In this lab we will use chemical reactions to generate hydrogen gas and oxygen gas. Be sure you understand how we will generate the gases by writing balanced chemical equations for the two reactions occurring in the gas generators. a. In one generator, solid zinc is added to aqueous hydrochloric acid to produce hydrogen gas and aqueous zinc chloride. b. In the other generator, liquid hydrogen peroxide (H2O2) decomposes in the presence of the catalyst, catalase, to form liquid water and oxygen gas. 2. The oxygen and hydrogen in the micro mole rocket will be ignited and will produce water vapor (along with some heat and light). Write a balanced chemical equation for the reaction. Analysis 1. Draw a bar graph for the relative loudness produced by pop-testing the various mixtures. Plot a second graph for plotting class averages. QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. 3. Explain your observations for the pop-test of pure H2 and of pure O2 (consider your answer to question 2 if you need help with this). 4. According to the pooled class data, what gas proportion (H2 : O2) produced the most explosive mixture? Why was that mixture the most explosive? Again, consider your answer to question 2. 5. Considering your answers to the above questions: if 0.001 mole of hydrogen gas reacts in your micromole rocket, how many moles of oxygen gas are consumed. 6. If 0.0224 liters of hydrogen react in your micromole rocket, how many moles of oxygen are consumed in the reaction? Going further: 7 . Why do the H2 and O2 in the collection bulb not react as soon as they mix? What role does the spark play? 6 . What methods did you attempt for making your rocket fly farther? Which ones worked?