BIOC 3653: Survey of Biochemistry
advertisement

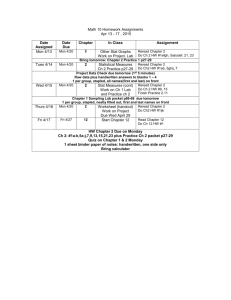
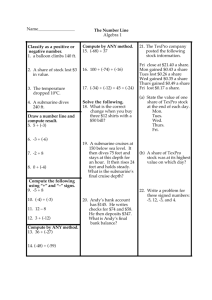
BIOC 3653: Survey of Biochemistry Spring 2011 Instructor: Dr. Ben Sandler 348G Noble Research Center 405-744-6194 benjamin.sandler@gmail.com Chief Teaching Assistant: Steven Pennington steven.pennington@okstate.edu Teaching Interns: John Paul Long Jon Downs Lecture: jplong@okstate.edu jon.downs@okstate.edu MWF 12:30-1:20PM, Animal Science 123 Office Hours: Ben Sandler: TR 9:00-10:20, 348G NRC, or by appointment. Review sessions TBA. Text: Biochemistry, 6th ed., Berg, Tymoczko, and Stryer. (Required.) “Skeleton Key” study guides, (by Dr. Sandler, free on the D2L site. Required.) The Secret Life of Equations, (by Dr. Sandler, free on the D2L site. Recommended.) Prerequisites: You are expected to have a working knowledge of algebra, general chemistry, and organic chemistry. You will need to be able to use logarithms, exponents, and scientific notation; to rearrange algebraic equations; to balance chemical equations; to understand the relationships between atomic number, valence, atomic structure, and reactivity; to calculate pH and use equilibrium constants and ionization constants; to know the properties (such as reactivity and polarity) of the major chemical functional groups; to know how to calculate concentration, among other skills. If you feel like you’re rusty on any of these, you must refresh your memory of these topics before the first exam. Note: you won’t be allowed to use a calculator on exams. Make sure you know how to calculate simple logarithms (such as log 0.01=?) with pencil and paper. I’ll put a logarithms study guide on the D2L site, in case your logarithm skills are rusty. D2L website: https://oc.okstate.edu The D2L site is an essential part of this class. It is your responsibility to check this site regularly for announcements, homeworks, grades, etc. The Powerpoint files for lectures will also be posted here. Keep your eye on the site as additional resources are added! Lecture Videos: Videos of the lectures will be available through the course website. (Instructions on how to view these videos will also be posted.) Homework: Homework problems will be regularly assigned and posted on the D2L website. As always, write clearly- unintelligible responses will receive a zero. These homeworks will be spot-graded (i.e., we will pick a subset of questions to grade on each homework,) and you will also receive some points for completing all the questions, even if your answers are incorrect. You are encouraged to work collaboratively on the homework. But, do not copy other people’s homework- even when you work together, your final answer must be your own, written in your own words. In order to be considered complete, answers to homework questions must show all your work, complete with your reasoning and calculations, and be presented in a neat and organized fashion. Remember, the goal is not merely to learn the material, but also to learn how to communicate your knowledge effectively. Exams: There will be four midterm exams, 50 minutes each, during the semester. You will need to bring your own orange Scantron sheet to each exam. Be sure to write clearlyunintelligible responses will get a zero! The lowest score on a midterm will be dropped. The final exam will be 2 hours long, and will cover information from the entire course. Makeup exams: There are no makeup exams in this class. Instead, if you miss an exam, it will be given the same percentage grade as your final exam grade. Grading Scheme: Your grade will be based on your performance on the homework (4 homeworks, at 20 points each,) the best 3 out of 4 midterms (160 points each,) and the final exam (240 points.) The total number of points possible is 800 points. (This figure does not include bonus points.) The assessment quiz does not count toward your grade. You must earn at least 720 points to make an A, at least 640 points to make a B, at least 560 points to make a C, and at least 480 points to make a D. Academic dishonesty and misconduct: Students are, as always, expected to follow the rules. Academic dishonesty and misconduct are defined in the OSU Policy and Procedures letter 2-0822. If you are found to have engaged in dishonesty or misconduct, the punishment will be at minimum an F on an assignment, up to and including an F in the course or worse. All exams are closed-book, closed-notes. Use of any notes, either on paper or on a calculator, is not permitted and will be considered cheating, and dealt with accordingly. Use of a calculator on an exam will also be considered cheating. Attendance: Attendance is strongly encouraged, but regular attendance is not required. For paperwork purposes, we will be taking roll every day starting on the second Wednesday, until roughly the midpoint of the semester. Only sign your own name! Disabilities: If you have special needs, please contact Dr. Sandler as soon as possible so that we can work with you and the Office of Disabled Student Services (326 Student Union) to provide reasonable accommodations for you. What to Study: You are only required to read the sections of the textbook that are covered in lecture. Exam questions will be taken from the lectures and Skeleton Keys, not from the textbook. How to Make an A in This Class: Biochemistry is very different from many of the subjects you’ve studied before. Before you could even begin to study biochemistry, you first had to study a great deal of biology and chemistry- particularly organic chemistry. Now, you’re going to need to combine your knowledge of biology and chemistry in order to understand biochemistry. Make sure you remember what you learned in your biology, general chemistry, and organic chemistry courses. I’ll give you an assessment quiz, so that you can figure out what topics you need to review. I’ll also post some review guides on the D2L site. Remember, too, that biochemistry is about narrative. For example, when your body breaks down foodstuffs, it does so in a series of steps. Understanding the biochemistry of metabolism means understanding what events happen, in what sequence, and why. Part of understanding that means understanding the basic principles involved, and being able to spot those concepts in different contexts. A good rule is this: if I mention a concept twice in class, in different contexts, then it will definitely be on the exam. Remember: merely being able to “plug and chug” an equation or memorize a graph will not enable you to do well in this class! It is essential that you genuinely understand what the material means. Many homework problems will require you to problem-solve, and that may include recognizing what extra information you need to have in order to solve the problem, and finding it online. And, most important of all, remember that the professor and teaching assistants are here to help you. If you’re finding biochemistry to be a challenging subject- and most people do- don’t hesitate to come to our office hours. We are genuinely eager to help you! Extra credit homework Two extra credit homeworks, each worth five points each, will be assigned during the semester. These will not be graded unless you are within 10 points of a grade cutoff. Student learning outcomes for BIOC3653 1. Students, given the Henderson-Hasselbalch equation, will able to perform pH calculations, predict the charge and protonation state of biomolecules in physiological solution, and describe their behavior and function in terms of their state. 2. Students will master the basic principles of protein structure. They will be able to recall, identify, and sketch all 20 amino acids, and classify them according to their properties, including the special properties of glycine, alanine, proline, and cysteine. They will be able to recall, define, and identify primary, secondary, tertiary, and quaternary structure. They will be able to recognize the alpha helix and beta sheet in representations of backbone traces and ribbon diagrams. 3. Students will master the basic principles of nucleic acid structure and function. They will be able to recall and identify the differences between purines and pyrimidines, and between bases, nucleosides, and nucleotides. They will be able to recall, list and describe the differences between DNA and RNA, and explain the ways these differences affect the function and behavior of nucleic acids. 4. Students will be able to produce a written explanation of how biological systems use randomness and selection to produce new information. They will be able to recognize features of biochemical systems as products of divergent or convergent evolution. 5. Students will master the principles of Gibbs Free Energy. Students will be able to make both quantitative and qualitative predictions of the outcome of a reaction, or of the distribution of conformational states of an enzyme, given either qualitative or quantitative information about the thermodynamics of the system. This includes being able to perform calculations involving ∆G, ∆Go, and ∆Eo, once given the equations. 6. Students will be able to determine and explain, in writing, where the energy comes from to drive enzymatic reactions or changes in protein conformation, using the “Ladder of Reactivity” and other chemical principles. 7. Students will be able to recall and explain in writing the significance of kcat, Vmax, and Km for enzyme kinetics. They will be able to recall and explain in writing the mechanisms of inhibition of competitive, uncompetitive, and noncompetitive inhibitors, and how these mechanisms result in their effects on the kinetic parameters. 8. Students will be able to recall and describe in writing, using standard conventions, the structures and roles of carbohydrates, lipids, and membranes in cells. They will be able to describe, in writing, how differences in the structures of these molecules result in differences in their properties and roles. 9. Students will be able to solve written problems using the core principles of metabolism and bioenergetics, in terms of reduction potentials. 10. Students will be able to recall, recognize, list, and explain important features held in common by a range of signal transduction pathways. 11. Students will be able to recall and explain in writing how carbohydrates, lipids, and proteins are broken down by the body, how energy is extracted from them, and how that energy is stored as ATP. 12. Students will be able to recall, list, and explain in writing the differences between lipid synthesis and breakdown. 13. Students will be able to recall and define in writing a number of important biochemical terms. Tentative Lecture, Quiz, and Exam Schedule, Spring 2011 DATE JAN. Mon. 10 Wed. 12 Fri. 14 Mon. 17 Wed. 19 Fri. 21 Mon. 24 Wed. 28 Fri. 28 Mon. 31 FEB Wed. 2 Fri. 4 Mon. 7 Wed. 9 Fri. 11 Mon. 14 Wed. 16 Fri. 18 Mon. 21 Wed. 23 Fri. 25 Mon. 28 MAR Wed. 2 Fri. 4 Mon. 7 Wed. 9 Fri.11 14-18 Mon. 21 Wed. 23 Fri. 25 Mon. 28 Wed. 30 APRIL Fri 1 Mon. 4 Wed. 6 Fri. 8 Mon. 11 Wed. 13 Fri. 15 Mon. 18 Wed. 20 Fri. 22 25-29 Mon. May 2 Topic Chapter Introduction. Assessment Quiz. Chemistry review. Protein composition and structure Protein composition and structure NO CLASS- MLK DAY Exploring proteins and proteomes DNA, RNA, and the flow of genetic information Exploring genes and genomes Exploring evolution and bioinformatics Hemoglobin: Portrait of a protein in action Exam 1 (Chapters 1-6) 1 2 2 Enzymes: Basic concepts and kinetics Enzymes: Basic concepts and kinetics Enzymes: Basic concepts and kinetics Catalytic strategies Regulatory strategies Carbohydrates Lipids and cell membranes Signal-transduction pathways Metabolism: Basic concepts and design Metabolism: Basic concepts and design Glycolysis and gluconeogenesis Exam 2 (Chapters 7-12, 14) 8 8 8 9 10 11 12 14 15, & pg 502-508 15, & pg 502-508 16 Glycolysis and gluconeogenesis Glycolysis and gluconeogenesis The citric acid cycle The citric acid cycle The citric acid cycle NO CLASS- SPRING BREAK Oxidative phosphorylation Oxidative phosphorylation Oxidative phosphorylation The light reactions of photosynthesis The light reactions of photosynthesis 16 16 17 17 17 Glycogen metabolism Exam 3 (Chapters 15-18) Glycogen metabolism Fatty acid metabolism Fatty acid metabolism Fatty acid metabolism Protein turnover and amino acid catabolism Protein turnover and amino acid catabolism Review of metabolism Exam 4(Chapters 19, 21-23) Dead week: Review FINAL EXAM 10:00-11:50 AM 21 3 4 5 6 7 18 18 18 19 19 21 22 22 22 23 23 HW HW 1 HW2 HW3 HW4