Sample Problems - PHARMACEUTICAL REVIEW
advertisement
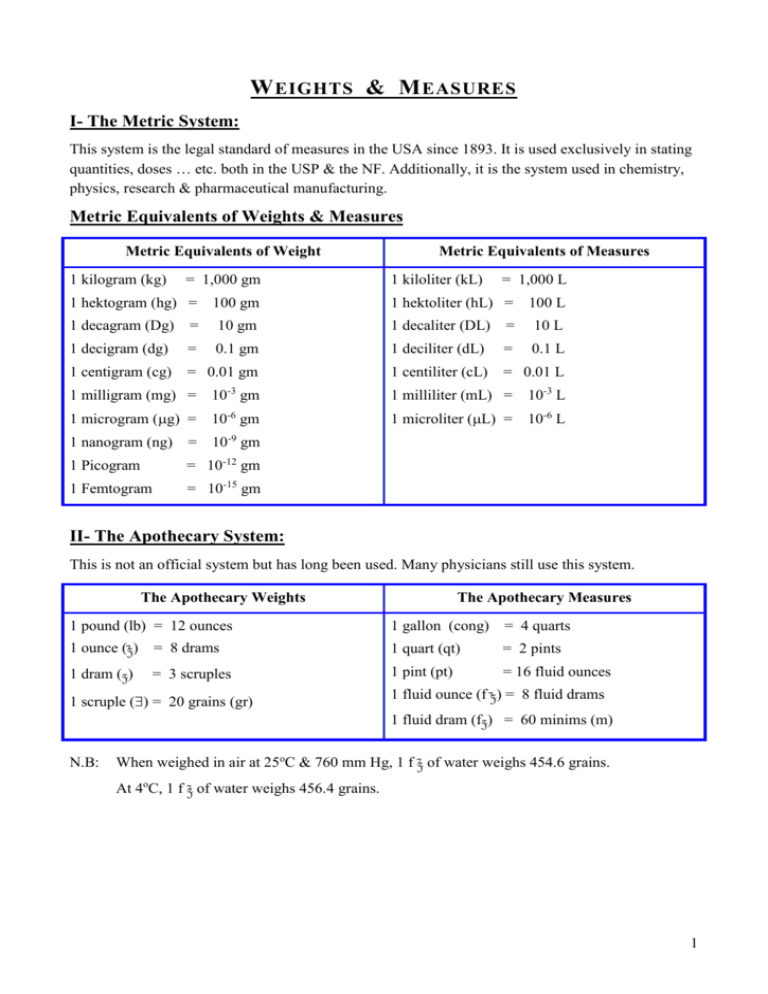
W EIGHTS & M EASURES I- The Metric System: This system is the legal standard of measures in the USA since 1893. It is used exclusively in stating quantities, doses … etc. both in the USP & the NF. Additionally, it is the system used in chemistry, physics, research & pharmaceutical manufacturing. Metric Equivalents of Weights & Measures Metric Equivalents of Weight 1 kilogram (kg) = 1,000 gm Metric Equivalents of Measures 1 kiloliter (kL) = 1,000 L 1 hektogram (hg) = 100 gm 1 hektoliter (hL) = 100 L 1 decagram (Dg) = 10 gm 1 decaliter (DL) = 10 L 1 decigram (dg) = 0.1 gm 1 deciliter (dL) = 0.1 L 1 centigram (cg) = 0.01 gm 1 centiliter (cL) = 0.01 L 1 milligram (mg) = 10-3 gm 1 milliliter (mL) = 10-3 L 1 microgram (g) = 10-6 gm 1 microliter (L) = 10-6 L 1 nanogram (ng) = 10-9 gm 1 Picogram = 10-12 gm 1 Femtogram = 10-15 gm II- The Apothecary System: This is not an official system but has long been used. Many physicians still use this system. The Apothecary Weights The Apothecary Measures 1 pound (lb) = 12 ounces 1 gallon (cong) = 4 quarts 1 ounce (z) = 8 drams 1 quart (qt) = 2 pints 1 dram (z) = 3 scruples 1 pint (pt) = 16 fluid ounces 1 scruple () = 20 grains (gr) N.B: 1 fluid ounce (f z) = 8 fluid drams 1 fluid dram (fz) = 60 minims (m) When weighed in air at 25oC & 760 mm Hg, 1 f z of water weighs 454.6 grains. At 4oC, 1 f z of water weighs 456.4 grains. 1 III- The Avoirdupois System: This system is used in buying & selling drugs & chemicals except for those which have been compounded in Rxs. E.g. Aspirin was sometimes bought by the avoirdupois system & dispensed on Rxs in the apothecary measures. The avoirdupois system has been used in large scale manufacturing of pharmaceuticals. The grain is the only weight which is identical in the apothecary & the avoirdupois systems of weight. The Avoirdupois Weights: 1 pound (lb) = 16 ounces 1 ounce (oz) = 437.5 grains (gr) IV- Metric Equivalence to the Apothecary & Avoirdupois Systems A- Metric Equivalence to the Apothecary System 1 ml = 16.23 minims 1 f z = 29.57 ml 1 gm = 15.432 gr 1 gr = 64.8 mg B- Metric Equivalence to the Avoirdupois System 1 pound (lb) = 453.4 gm (~ 454 gm) 1 kg = 2.2 lb V- House Hold Measures 1 tea spoonful = 5 ml 1 measuring cup = 240 ml 1 dessert spoonful = 10 ml One glassful = 240 ml 1 table spoonful = 15 ml One wine glassful = 60 ml 1 tea cupful = 120 ml 1 ml = 32 drops VI- Sample Problems: A. Conversions: 1. How many tablets, each containing 25 g, will furnish the equivalent of 0.5 mg of drug A? 0.5 mg = 0.5 X 1000 = 500 g Number of tablets = 500 / 25 = 20 tablets. 2. How many grams of thiamine HCl should be used to prepare 500 tablets each containing 200 g of active drug? Active drug needed = 200 X 500 = 100, 000 g = 100,000/1,000,000 = 0.1 g 3. A formula specifies a conc. of 0.625 gm in a 2.5 liters, what is the conc. in mg/ml? 0.625 gm = 0.625 X 1000 = 625 mg 2.5 liters = 2.5 X 1000 = 2500 ml conc. in mg/ml = 625 / 2500 = 0.25 mg/ml 2 4. Add the following volumes: 5 gal, 3 pt, 2 f z, and 2 pt, 3 f z, 4 fz. Gal pt f z fz 5 3 2 5 2 3 5 --4 4 Sum = 5 But 2pt = 1 qt Thus, the correct answer is 5 gal, 2 qt, 1pt, 5 fl oz, 4 fl dr. 5. Add the following weights: 4 lb, 3 z, 1 z, 59 gr, and 5 lb, 10 z, 7 z, 2 gr. Sum = lb z z gr 4 3 1 59 5 10 7 2 9 13 8 61 9 13 9 1 9 14 1 1 1 1 10 2 Thus, the final answer is 10 lb, 2 z, 1 z, 1gr 6. How much is left in a 5 L container after the removal of 895 ml? Amount remaining = 5000 – 895 = 4, 105 ml 7. A pharmacist buys 1 oz of drug C. At varying intervals the following amounts are withdrawn from that supply z ii, z ss, ii , 56 gr, 48 gr. What is the amount remaining? 1 oz = 437.5 gr (Avoir du pois) Amounts withdrawn = 2 X 60 = 120 gr + (1 z = 60 gr) 0.5 X 60 = 30 gr + (1 = 20 gr) 2 X 20 = 40 gr + 56 gr + 48 gr = Amount remaining = 437.5 – 294 = 143.5 gr 294 gr 8. If a drug costs $ 1.75 per oz, what is the cost of 2 z? 1 oz = 437.5 gr and cost $ 1.75 2 z = 2 X 60 = 120 gr 437.5 $1.75 120 ?? Thus the cost of 2 z = 120 X 1.75 / 437.5 = $ 0.48 = 48 c. 9. Convert fl z ii ss to ml. fl z = 29.57 ml fl z = 8 fl z Thus fl z = 29.57 /8 = 3.69 ml 2.5 fl z = 3.69 X 2.5 = 9.225 ml. Another solution: fl z = 60 mimims 1 ml = 16. 23 minims 2.5 fl z = 2.5 X 60 / 16.23 = 9.24 ml. 3 B. Dose Calculations: 1. How many tsp would be prescribed in each dose of a medicine of fl z vi contained in 16 doses? 6 fl z = 6 X 29.57 = 177.42 ml No of tsp in 6 fl z = 177.42 / 5 = 35.48 tsp No of tsp prescribed in each dose = 35.48 / 16 = 2.2 tsp 2. How many drops would be prescribed in each dose of a medicine if 15 ml contains 60 doses? The dispensing dropper calibrates 32 drops / ml. Thus 15 ml = 15 X 32 = 480 drops No of drops / dose = 480/ 60 = 8 drops 3. How much medicine is needed to provide 2 tablespoonfuls twice daily for 8 days? 2 tablespoonfuls = 2 X 15 = 30 ml Daily dose = 30 X 2 = 60 ml Amount of drug needed for 8 days = 8 X 60 = 480 ml 4. How many mgs of a drug are needed to prepare 72 doses of 1/12 gr each? No of gr needed = 1/12 X 72 = 6 gr No of mg needed = 6 X 64.8 = 388.8 mg 5. If 125 tablets contain 0.05 gm of drug X, how much is contained in 1 tablet? 1 tablet contains = 0.05 X 1000 / 125 = 0.4 mg = 0.4 X 1000 = 400 g 6. Given the Rx below, calculate the amount of each medication in one dose. Rx Codeine phosphate 0.6 g Ammonium chloride 6.0 g Cherry syrup ad 120 ml sig 1 tsp t.i.d. No of doses = 120 / 5 = 24 dose Amount of Codeine phosphate / dose = 0.6 X 1000 / 24 = 25 mg Amount of Ammonium chloride / dose = 6 X 1000 / 24 = 250 mg 7. How many grams of medicine are needed to make 120 ml of a solution in which each tsp will contain 3 mg of the medicine? 5 ml 3 mg 120 ml ?? 120 ml will contain = 120 X 3 / 5 = 72 mg = 72/1000 = 0.072 gm of medication 8. The dose of a drug is given as 3mg/kg body wt., how much medication should be given to a patient weighing 154 lb? Patient weight = 154 X 453.6 / 1000 = 70 kg The dose = 70 X 3 = 210 mg 9. For penicillin V, if 1 unit = 0.6 mg, how many mg are there in 500,000 unit: a. 200 mg c. 300 mg b. 250 mg d. 500 mg 4 10. A powder is divided into 36 capsules, if each contains 0.5 mg of drug X and 15 mg of drug Y and enough of Z to make 0.3 gm capsule. How much is there of each in the original powder. Amount of drug X in 36 capsules = 36 X 0.5 = 18 mg Amount of drug Y in 36 capsules = 36 X 15 = 540 mg Amount of Z in 36 capsules = 0.3 X 36 – (18 + 540 / 1000) = 10.242 gm 11. If the dose of a medicine is 5 mg / kg body wt., how is the dose expressed in mg / pound? 1 kg = 2.2 lb Thus dose = 5 / 2.2 = 2.27 mg / lb 12. How many capsules of 250 mg each should be used to prepare 100 ml of a solution that has a conc. of 250 mg / 5 ml: a. 5 capsules c. 10 capsules b. 20 capsules d. 25 capsules C. Pediatric Doses: 1. Young’s Rule: Child dose = [Age X Adult dose] / (Age + 12) 2. Couling’s Rule: Infant dose = [Age (at next birthday) X Adult dose] / 24 3. Fried’s Rule: Infant dose = [Age (in months) X Adult dose] / 150 4. Clark’s Rule: Child dose = [Weight (in lbs) X Adult dose] / 150 Sample Problems: 1. If the adult dose of X is 5 mg, what is the dose of a child of 8 yrs using Young’s law? Child dose = [8 / 8 + 12] X 5 = 2 mg 2. If the adult dose of Y is 325 mg, what is the dose of Y for a 6 year child using Young’s law? Child dose = [6 / 6 + 12] X 325 = 108.33 mg 3. If the adult dose of Z is 0.6 mg, what is the dose for a child weighing 45 lbs using Clark’s law? Child dose = 45 X 0.6 / 150 = 0.18 mg = 180 g 4. The adult dose of a drug is 500 mg, what is the dose of a child of 12 months using Fried’s law? Child dose = 12 X 500 / 150 = 40 mg 5. If the adult dose of a medicine is 250 mg, what is the dose of a child of 20 months? Fried’s law Child dose = 20 X 250 / 150 = 33.3 mg Couling’s law Child dose = 2 X 250 / 24 = 20.8 mg 6. A physician prescribed 250 mg of tetracycline capsules for an adult weighing 165 lb & specified that he wants 25 mg/kg body wt. per day for 12 days. He ordered a similar Rx for a child of 8 yrs. Basing your calculations on Young’s rule, how many capsules of tetracycline 250 mg will be dispensed in filling the 8 years child Rx for 12 days? Adult daily dose = 165 X 25 / 2.2 = 1875 mg Adult dose for 12 days = 1875 X 12 = 22,500 mg = 90 capsules Child’s dose = 90 X 8 / 12 + 8 = 36 capsules 5 D. Reducing & Enlarging Formulae: 1. Given the following Rx Benzyl benzoate 250 mg Triethanol amine 5.00 gm Oleic acid 20.0 gm Aq. dist. ad q.s. to 1000 ml Make 180 ml of this formula. The factor is 1000/180 = 5.55 Thus the formula will be 2. Given Benzyl benzoate = 250 / 5.55 = 45 mg Triethanol amine = 5 / 5.55 = 0.9 mg Oleic acid 20 / 5.55 = 3.6 gm Aq. dist. ad q.s. to 180 ml Green soap 120 gm Comphor 45 gm Alcohol 700 ml Aq. dist. q.s. ad 1000 ml Make 5 gallons of this formula. The factor is 5 X 3784 / 1000 = 18.92 The formula will be Green soap = 120 X 18.92 = 2,270 gm = 2.27 kg Comphor = 45 X 18.92 = 851.4 gm Alcohol = 700 X 18.92 = 13,244 ml = 13.244 L Aq. dist. q.s. ad 5 gallons E. Formulae Specifying Proportional Parts: 1. Given the following formula, calculate the quantity of ingredients needed to make 1000 gm. X 5 parts Y 10 parts Z 50 parts The total parts = 5 + 10 + 50 = 65 parts Since 65 parts = 1000 gm , thus 1 part = 1000 / 65 = 15.385 gm Thus, X 5 X 15.385 = 76. 925 gm Y 10 X 15.385 = 153.85 gm Z 50 X 15.385 = 769.25 gm 6 VII- Density, Specific Gravity & Specific Volume: A. Density: is defined as the mass of a substance per unit volume of the substance. Thus Density (D) = Mass (M) / Volume (V), D=M/V Water weighs 10 gm for each 10 ml, thus the density of water = 10/10 = 1 For conc. H2SO4, 10 ml weigh 18 gm, thus the density of conc. H2SO4 is 18/10 = 1.8 N.B: Density is a concrete number that does change with external conditions, thus it must change as the units of measurement change. B. Specific Gravity: It is the ratio of the weight of a mass to the weight of an equal volume of a standard substance. Water is the standard for liquids & solids. Thus sp. gr. = Weight of X ml of substance / Weight of X ml of water i.e. Sp. gr. = Wt / V For conc. H2SO4, if 10 ml weigh 18 gm, thus the sp. gr. = 18 / 10 = 1.8 Specific gravity is an abstract number, thus it will never change regardless of units of measurement used. C. Specific Volume: It is the decimal ratio of the volume of a substance to the volume of an equal weight of another standard substance, usually water. Thus Sp. volume = Volume of X gm of substance / Volume of X gm of water i.e. Sp. vol = V / Wt Sample Problems: 1. If 91 ml of a liquid weigh 107.16 gm, calculate the specific volume. Specific volume = Volume of X gm of substance / Volume of X gm of water = 91 / 107.16 = 0.849 2. If the sp. gr. of a substance is 1.71, what is its specific volume? Sp. gr. = Weight of X ml of substance / Weight of X ml of water Thus the weight of 10 ml of the substance = 1.71 X 10 = 17.1 gm The sp. volume of the substance = Volume of X gm of substance / Volume of X gm of water = Volume of 17.1 gm of substance / Volume of 17.1 gm water = 10 / 17.1 = 0.585 In other words, the sp. volume is the reciprocal of the sp. gr. i.e. sp. volume = 1 / sp. gr 3. If a liquid has a sp. volume of 1.396, calculate its sp. gr. Sp. gr. = 1 / sp. volume = 1 / 1.396 = 0.716 4. If 250 ml of alcohol weigh 203 gm, what is its density? Density = M / V = 203 / 250 = 0.812 gm/ml 5. If a piece of metallic copper weighs 53.6 gm & displaces 6 ml of water, what is its density? Since the volume of the piece of copper = the volume of water displaced, thus Density = M / V = 53.6 / 6 = 8.933 gm/ml 6. If 1200 ml of a liquid weigh 1125 gm, what is its sp. gr.? Sp. gr. = Weight of X ml of substance / Weight of X ml of water = 1125 / 1200 = 0.938 7 7. If 500 ml of solution X weigh 650 gm, what is its sp. gr.? Sp. gr. = 650 / 500 = 1.3 8. If 1L of solution weighs 1313 gm, what is its sp. gr. & its sp. volume? Sp. gr. = 1313 / 1000 = 1.313 Sp. volume = 1000 / 1313 = 0.762 9. If 1 pint of liquid weighs 558 gm, what is its specific gravity? 1 pint = 16 fl z = 16 X 29.57 = 473 ml Sp. gr. = 558 / 473 = 1.18 10. What is the % w/v of 60% sulfuric acid (sp. gr. = 1.28)? % w/v = 60 X 1.28 = 76.8 g% w/v 11. 30 ml of glycerol in 150 ml soln (sp. gr. of glycerol is 1.25), what is the % w/v of glycerol? Wt of glycerol = 30 X 1.25 = 37.5 gm % w/v = 37.5 / 150 = 25 g% w/v D. Calculating the Weight of an Unknown Liquid, Given its Volume & Specific Gravity: The sp. gr. equation states that: sp. gr. = Weight of X ml of substance / Weight of X ml of water Given the specific gravity & the volume of the unknown liquid, one can calculate its weight Wt. of the liquid = sp. gr. of liquid X weight of an equal volume of water Wt. of the liquid = sp. gr. of liquid X volume of the liquid 1. What is the wt. of 3620 ml of alcohol given its sp. gr. is 0.82? Wt. of alcohol = 3620 X 0.82 = 2,968.4 gm 2. If an unknown solution has a sp. gr. of 1.16, calculate the weight of 100 ml of this solution. Wt of 100 ml = 100 X 1.16 = 116 gm 3. What is the weight in gm of 2 fz of a liquid whose sp. gr. is 1.118? 2 fz = 2 X 29.57 = 59.14 ml Thus the weight of 2 fz of this liquid = 59.14 X 1.118 = 66.12 gm 4. What is the weight of 225 ml of a liquid that has a sp. gr. of 1.83? Wt = 225 X 1.83 = 411.75 gm 5. Ether has a sp. gr. of 0.715, what is the wt in grs of 50 ml of ether? Wt of 50 ml of ether = 0.715 X 50 = 35.75 gm Since 1 gm = 15.432 gr, thus, the wt of 50 ml of ether = 35.75 X 15.432 = 551.69 gr. 6. What is the % w/w of absolute alcohol in a solution of alcohol in water having a sp. gr of 0.852 & containing 65% v/v alcohol, given that the sp. gr. of alcohol is 0.798? Absolute alcohol in 100 ml of the 65% solution = 65 ml = 65 X 0.798 = 51.87 gm 100 ml of the solution weigh = 100 X 0.852 = 85.2 gm Thus the % w/w of alcohol in this solution = 51.87 X 100 / 85.2 = 60.88% w/w E. Calculating the Volume of an Unknown Liquid, Given its Weight & Specific Gravity: 8 From the sp. gr. equation, Since Specific Gravity = Weight of X ml of substance / Weight of X ml of water = Wt of X ml of substance / Volume of substance Thus, Volume of a liquid = Wt of liquid / sp. gr. 1. An unknown liquid has a sp. gr. 1.71, calculate the volume of 100 gm of this liquid. Volume = Wt / Sp. gr. = 100 / 1.71 = 58.48 ml 2. What is the volume of 492 gm of HNO3 if its sp. gr. is 1.4? Volume = 492 / 1.4 = 351.43 ml 3. What is the volume of a substance weighing 1000 gm if its sp. gr. is 1.83? Volume = 1000 / 1.83 = 546.45 ml 4. A Rx calls for 425 gm of a material whose sp. gr. is 1.155. How many ml must be dispensed? ml to be dispensed = 425 / 1.155 = 367.97 ml 5. What is the volume in f z of 1 lb of methyl salicylate given its specific gravity is 1.185? Volume of 1 lb = 454 / 1.185 = 383.12 ml = 383.12 / 29.57 = 12.96 f z VIII- Percentage Calculations: There are 3 types of percentages: A. Weight / Weight Percentage (w/w %): These are represented as number of grams of constituent per 100 gram of solution or mixture. B. Weight / Volume Percentage (w/v %): These are represented as number of grams of constituent per 100 ml of solution. C. Volume / Volume Percentage (v/v %): These are represented as number of ml of constituent per 100 ml of solution. 1. How many gm of X are required to prepare 250 ml of a 5% w/v solution? No of gm needed = 5 X 250 / 100 = 12.5 gm 2. How many grams of a chemical are required to make 120 ml of a 20% w/w solution, given the sp. gr. of the solution is 1.15? Wt 120 ml of the solution = 120 X 1.15 = 138 gm Grams of solute needed for 120 ml of solution (20% w/w) = 138 X 20 / 100 = 27.6 gm 3. How many grams of phenol are needed to make 250 gm of 5% w/w aqueous solution? Grams of phenol = 5 X 250 / 100 = 12.5 gm 4. In the following Rx how many mls of liquefied phenol are required? 9 Rx liquefied phenol 2.5% Boric acid solution 240 ml Sig. apply externally as directed. ml of liquefied phenol = 2.5 X 240 / 100 = 6 ml 5. How many minims of wintergreen oil should be used in compounding the following Rx Rx Wintergreen oil 5% Isopropyl alc. ad f z iv Sig. apply externally as directed. Volume of solution = 4 X 29.57 = 118.28 ml Volume of winter green oil needed = 5 X 118.28 / 100 = 5.914 ml = 5.914 X 16.23 = 95.98 minims 6. How many grains of atropine sulfate are needed to compound the following Rx? Rx Atropine sulfate 2% Dist. water q.s. ad fz iv Volume of solution = 4 X 29.57 / 8 = 14.78 ml Weight of atropine sulfate needed = 14.78 X 2 / 100 = 0.296 gm = 0.296 X 15.432 = 4.56 gr 7. If 80 ml of a solution contain 12 g of urea what is the conc. % of urea? Conc. % = 100 X 12 / 80 = 15% w/v 8. How much dextrose is required to make 400 ml of 5% dextrose? Dextrose needed = 400 X 5 / 100 = 20 gm 9. How many f z of 10% solution could be made from 182 grains of silver nitrate? 182 gr silver nitrate = 182 X 64.8 = 11793.6 mg = 11.79 gm Volume of 10% solution that can be prepared = 11.79 X 100 / 10 = 117.9 ml = 117.9 / 29.57 = 3.988 f z 10. How many ml of a 3% solution of ephedrine sulfate can be made from 27 gm of active ingredient? Volume of 3% solution = 27 X 100 / 3 = 900 ml 11. A solution of a drug is 1 : 10,000, what is the % w/v in mg? x= 1 10,000 x 100 100 X 1 / 10,000 = 0.01gm = 0.01 X 1000 = 10 mg IX- Ratio Calculations: Ratios are expressed in the following units: 10 A. For Solids in Liquids: Grams / 1000 ml of solution B. For Liquids in Liquids: Milliliters / 1000 ml of solution C. For Solids in Solids: Grams / 1000 Gram of solution Grains / 1000 grains of solution 1. Express 0.02% as a ratio strength. 0.02 / 100 = 1 (part) /?? parts 2/ 10,000 = 1 / 5,000 2. Express 1 : 4,000 as a % concentration. 1 / 4000 = ?? / 100 = 0.025% 3. A Rx calls for a solution of drug of 2 mg / ml. Express the conc. of this Rx as a ratio strength. 2 mg / ml = 2000 mg / 1000 ml = 2 gm / 1000 ml = 1 : 500 Another solution: 0.002 gm / 1 ml = 1gm / X ml X = 500 Thus, the ratio is 1 : 500 4. What is the ratio strength of the solution made by dissolving 2 tablets each of 125 mg mercury bromide in 500 ml of solvent? 125 X 2 = 520 mg = 0.25 gm The ratio is 0.25 : 500 i.e. 1 : 2000 5. How many grains of K permanganate could be used to make 500 ml of 1 : 2500 solution? Amount of K permanganate needed = 1 X 500 / 2500 = 0.2 gm = 0.2 X 15.432 = 3.086 grains 6. How much gentian violet is used to make 500 ml of 1 : 10,000 solution? Amount needed = 500 X 1 / 10,000 = 0.05 gm = 50 mg 7. A nurse asks for assistance. She has a 10 ml vial of thyroxine 1 : 50, which she has to use to make a 0.05% solution. What is the volume of solution she can make? Conc. of the vial = 100 X 2 / 50 = 2% Thus, 2 X 10 = 0.05 X ??? Volume of the 0.05% solution she ca prepare = 2 X 10 / 0.05 = 400 ml Another solution: 10 ml vial contains = 10 / 50 = 0.2 gm thyroxine The volume of solution she can make = 0.2 X 100 / 0.05 = 400 ml 8. How many ml of K permanganate 1:500 are needed to prepare 120 ml of a soln. so that 5 ml diluted to 500 will produce 1 : 2500 solution? 1 : 2500 = 1 X 100 X 1000 / 2500 = 0.04 g% Conc. of the needed soln = 0.04 X 500 / 5 = 4 gm % 120 ml will need = 120 X 4 / 100 = 4.8 gm ml of 1 : 500 needed = 4.8 X 500 / 1 = 2400 ml X- Dilution & Concentration of Solutions: There are 2 important rules to follow: 11 A. When ratio strengths are given in the problem, convert to percentage (%) strengths before beginning your calculations. B. Whenever the problem deals with proportion parts, reduce them to the lowest possible common denominator. 1. If 500 ml of 15% v/v solution is diluted to 1500 ml, what is the % strength of final solution? Since: Strength X Volume = Strength X Volume Thus 15 X 500 = ?? X 1500 The % strength of the final solution = 15 X 500 / 1500 = 5% 2. If 20 ml of a 1 : 200 w/v solution are diluted to 500 ml, what will the final strength be? The strength of the original solution = 1/200 = 0.5 % Thus 0.5 X 20 = ?? X 500 The strength of the final solution = 0.5 X 20 / 500 = 0.02% = 0.02 : 100 = 1 : 5000 3. If a 65% w/v sugar solution is evaporated to 85% of its original volume, what will be its conc? Thus, 65 X 100 = ?? X 85 The strength of the resulting solution is 65 X 100 / 85 = 76.47% 4. How many ml of 1:5000 K permanganate solution can be made from 50 ml of 0.5% solution? 1 : 5000 K permanganate = 0.02% solution Thus, 50 X 0.5 = ?? X 0.2 The volume of solution that can be made = 50 X 0.5 / 0.02 = 1250 ml 5. You receive the following Rx Ephedrine sulfate 0.25% Rose water ad 10 ml How many ml of 1 : 50 stock solution of ephedrine sulfate are necessary for dispensing? Conc. of stock solution = 1 : 50 = 2% Thus 0.25 X 10 = 2 X ?? Amount of Stock solution to be used = 0.25 X 10 / 2 = 1.25 ml 6. A preservative solution contains 21.3% w/v benzalkonium chloride. What volume of this solution is required to produce 1 quart of 1 : 10,000 solution? 1 quart = 2 pints = 32 f z = 32 X 29.57 = 946.24 ml 1 : 10,000 solution = 100 X 1 / 10,000 = 0.01% 0.01 X 946.24 = ??? X 21.3 Volume of 23.1% solution needed = 0.01 X 946.24 / 21.3 = 0.444 ml 7. A hospital clinic requests 2 lb of 2% hydrocortisone ointment. How man gm of 5% HC ointment will be diluted with white petrolatum to prepare this order: 2 X 909 gm = 5 X ???? Amount of 5% HC ointment needed = 2 X 909 / 5 = 363.6 gm 8. How much alcohol 95% will you need to prepare the following Rx Rx Alcohol 65% 12 Water 100 65 X 12 = 95 X ???? ml of 95% alcohol needed = 65 X 12 / 95 = 8 ml XI- Alligation: 12 This is an arithmetic method of solving problems that involve the mixing of solutions, ointments, mixtures of solids, etc. possessing different percentage strengths. Alligation is used to calculate the % strength of a mixture made by mixing 2 or more components of a given % strength. 1. What is the % v/v of alcohol in the following mixture: 1 L 60%, 3L 40%, 1000 ml 70%? 1000 X 60% = 600 ml absolute alcohol 3000 X 40% = 1200 ml absolute alcohol 1000 X 70% = 700 ml absolute alcohol Thus 5000 2500 ml absolute alcohol Thus 100 ml contain ??? Concentration = 100 X 2500 / 5000 = 50% 2. What is the final % of ZnO ointment made by mixing ZnO ointments of the following strengths: 200 gm of 10%, 50 gm of 20% and 100 gm of 5%. 200 X 10% = 20 gm ZnO 50 X 20% = 10 gm ZnO 100 X 5% = 5 gm ZnO Thus 350 gm contain 35 gm ZnO i.e. final % of ZnO ointment = 35 / 350 = 10% N.B: Occasionally you might run in a problem where the addition of a diluent or solvent is included. In such cases consider the volume of the diluent as having 0% conc. of the active drug. XII- Alligation Alternate: To find out the relative amounts of solute or other substances of different strengths you must use the X formula to make a mixture of the required strength. High conc. ingredient Part of high conc. ingredient needed Desired conc. Low conc. ingredient Part of low conc. ingredient needed 1. Which proportion of 95% & 50% alcohol should be used to make 70% alcohol solution? 95% 20 parts 70% 50% 25 parts The final proportions are 20 : 25 N.B: This system can be used to determine the relative concentrations of 3,4 or more different strengths required to prepare another requisite strength. 2. In what proportion should 15% boric acid solution be mixed with white petrolatum to produce 2% boric acid ointment. 15% 2 parts of 15% boric acid solution 2% 0% 13 parts of white petrolatum Thus the final proportions are 2 : 13 13 3. A pharmacist wishes to prepare a 10% ointment of drug X, he has some 50%, 20% & 5% in stock. In what proportions should he mix these to make the 10% product? 50% 5 parts of 50% 10% 5% 40 parts of 5% 20% 5 parts of 20% 10% 5% 10 parts of 5% The total is 5 parts of 50% + 5 parts of 20% + 50 parts of 5%. 4. In what proportions should the following strengths be mixed to attain a 10% mixture 20%, 15%, 5% and 3% ? 20% 15% 7 parts of 20% 5 parts of 15% 10% 5% 3% 5 parts of 5% 10 parts of 3% 5. How much ZnO powder should be added to 300 gm of 20% ZnO ointment to produce an ointment containing 25% ZnO? 100% 5 parts of 100% 25% 20% 75 parts of 20% Thus every 75 parts of 20% ointment need 5 parts of ZnO powder to yield a 25% ointment. Thus, the amount of ZnO powder needed for 300 g 20% ointment = 300 X 5 / 75 = 20 g 6. How much 30% alcohol, 45% alcohol, 60% alcohol & 95% alcohol, could be mixed to make 200 ml of 50% alcohol? 30% 45 parts of 95% alcohol 45% 10 parts of 45% alcohol 50% 60% 5 parts of 60% alcohol 95% 20 parts of 95% alcohol Thus the total number of parts = 80 parts Amount of 30% alcohol needed to prepare 200 ml 50% alcohol = 45 X 200 / 80 = 112.5 ml Amount of 45% alcohol needed to prepare 200 ml 50% alcohol = 10 X 200 / 80 = 25 ml Amount of 60% alcohol needed to prepare 200 ml 50% alcohol = 5 X 200 / 80 = 12.5 ml Amount of 95% alcohol needed to prepare 200 ml 50% alcohol = 20 X 200 / 80 = 50 ml 7. A cream requires 5 gm of an emulsifying blend of span 80 & tween 80. If the required HLB is 10.5, how many gm of each should be used. HLB of span 80 = 4.3; HLB of tween 80 = 15: Span 80 4.3 4.5 parts 10.5 Tween 80 15 6.2 parts Total parts = 10.7 parts Gm of Span = 4.5 X 5 / 10.7 = 2.1 gm Gm of Tween = 6.2 X 5/ 10.7 = 2.9 gm 14 8. A physician requires an elixir containing 32% alcohol. How much low alcohol elixir (10%) & high alcohol elixir (78%) must be mixed to prepare 1 pint of the desired conc.: Low alcohol elixir 10% 46 parts 32% High alcohol elixir 78% 22 parts Total parts = 68 parts 1 pint = 16 fl oz = 16 X 29.57 = 473 ml Volume of low-alcohol elixir needed = 46 X 473 / 68 = 320 ml Volume of high-alcohol elixir needed = 22 X 473 / 68 = 153 ml XIII- Temperature Conversion: All problems of conversion of Fahrenheit (oF) to Centigrade (oC) temp. are solved by the formula: 9 X oC = 5 X oF -160 1. Convert 32 oF to centigrade. 9 X oC = 5 X 32 -160 Thus oC = (5 X 32) – 160 / 9 = 0 / 9 = 0 oC 2. Convert 212 oF to oC. 9 X oC = (5 X 212) – 160 = 900 Thus oC = 900 / 9 = 100 oC 3. Convert 100 oC to oF. 9 X oC = 5 X oF – 160 9 X 100 = 5 X oF -160 o F = 900 + 160 / 5 = 212 oF XIV- Proof Spirits: One proof gallon is defined as the gallon of 100 proof (50%) ethyl alcohol. Proof Gallon = Wine gallon X Proof strength / 50% Any quantity of alcohol containing the equivalent of 1 gallon of 50% alcohol is a proof gallon. Thus ½ gallon of 200 proof (100%) alcohol is a proof gallon; similarly, 2 gallons of 25% alcohol is a proof gallon. The wine gallon is the common unit of volume measure. 1. How many proof gallons are there in 55 gallons of 45% alcohol? Proof gallons = gallons X strength / 50 = 55 X 45 / 50 = 49.5 P.G. 2. How many proof gallons are there in 25 gallons of 70% alcohol? Proof gallons = 25 X 70 / 50 = 35 P.G. 15 XV- Isotonic Solutions: When a solvent passes through a semi-permeable membrane, from a dilute solution into a more concentrated solution, concentrations tend to become equalized. This phenomenon is called osmosis and the pressure responsible for such a phenomenon is known as the Osmotic Pressure. It is generally agreed that solutions mixed with body fluids should have the same osmotic pressure as these fluids, i.e., they should be isotonic with these fluids. Standard solutions known to be isotonic with body fluids are: Sodium chloride 0.90% Dextrose 5.00% Boric acid 1.73% In general, most solutions of drugs are made isotonic by adding NaCl. The amount of NaCl that must be added is calculated by the following process: a. Calculate the amount of NaCl (in gm or gr) represented by the ingredients of the Rx. This is done by multiplying the amount of each ingredient (in gm or gr) by its NaCl equivalent. b. Calculate the amount of NaCl alone (in gm or gr) that would be contained in an isotonic solution of the same volume (0.9% or 0.009 gm / ml). c. Subtract the total amount of NaCl represented by ingredients (from step 1) from the total amount required (from step 2) and the difference will thus represent the total amount of NaCl needed. d. If an agent other than NaCl is to be added to make the solution isotonic, as dextrose or boric acid, divide the final amount of solution required by its NaCl equivalent. e. If water is to be added beside NaCl, then dilute with X ml of water and complete with q.s. NaCl. 1. How much NaCl must be added to the following Rx to make it isotonic? Rx ZnSO4 1/4 % Phenyl ephrine 1/8 % NaCl q.s. Aq. dist. ad to N.B: 30 ml NaCl equivalent of ZnSO4 0.16 NaCl equivalent of phenyl ephrine 0.29 Zn SO4 required = 0.25 X 30 / 100 = 0.075 gm Phenyl ephrine required = 0.125 X 30 / 100 = 0.0375 gm NaCl equivalent to ZnSO4 = 0.075 X 0.16 = 0.012 NaCl equivalent to phenyl ephrine = 0.0375 X 0.29 = 0.01 Thus, total NaCl Equivalent = 0.012 + 0.01 = 0.022 gm. NaCl needed if used alone = 0.9 X 30 / 100 = 0.27 gm Amount of NaCL needed for the formula = 0.27 – 0.022 = 0.248 gm 16 2. How much NaCl is needed to make the following Rx isotonic? Rx Ephedrine sulphate gr iv NaCl q.s. Ft soln so f z i Given NaCl equivalent 0.2 NaCl equivalent to ephedrine SO4 = (4 X 64.8 /1000) X 0.2 = 0.052 gm NaCl needed if used alone = 29.57 X 0.9/100 = 0.266 gm NaCl needed to make the Rx isotonic = 0.266 – 0.052 = 0.214 gm 3. How much boric acid is needed for the following ophthalmic preparation? Rx Halocaine HCl 1% (NaCl equiv. = 0.17) Chlorobutanol 0.5% (NaCl equiv. = 0.18) Boric acid q.s. (NaCl equiv. = 0.52) Aq. dist. ad to 60 ml Fiat isotonic solution NaCl equivalent to halocaine HCl = 1 X 60 X 0.17 / 100 = 0.102 NaCl equivalent to Chlorobutanol = 0.5 X 60 X 0.18 / 100 = 0.054 NaCl needed if used alone = 60 X 0.9 / 100 = 0.54 gm NaCl needed to make the solution isotonic = 0.54 – 0.102 – 0.054 = 0.384 gm Since 1 part boric acid Thus is equivalent to 0.52 NaCl ?? 0.384 gm Boric acid needed to make the solution isotonic = 0.384 X 1 / 0.52 = 0.738 gm 4. How much NaCl is required for the following Rx Silver nitrate 1 : 500 Ft isotonic solution 60 ml (NaCl equiv. = 0.34) Silver nitrate needed = 1 X 60 / 500 = 0.12 gm NaCl equivalent to AgNO3 = 0.12 X 0.34 = 0.041 gm NaCl needed if used alone = 60 X 0.9 / 100 = 0.54 gm NaCl needed to make the solution isotonic = 0.54 – 0.041 = 0.499 gm 5. How much NaCl is needed to adjust the isotonicity of the following Rx? ZnSO4 1% NaCl q.s. Purified water to 60 ml (NaCl equiv. = 0.15) ZnSO4 needed for the Rx = 60 X 1 / 100 = 0.6 gm NaCl equivalent to ZnSO4 = 0.6 X 0.15 = 0.09 gm NaCl needed if used alone = 60 X 0.9 / 100 = 0.54 gm NaCl needed to make the solution isotonic = 0.54 – 0.09 = 0.45 gm 17 6. 25 ml of 0.5% solution of drug X (NaCl equivalent = 0.31), how many mg of NaCl are needed to render the solution isotonic? Amount of drug in 25 ml = 0.5 X 25 X 1000 / 100 = 125 mg NaCl equivalent = 125 X 0.31 = 38.75 mg NaCl needed if used alone = 25 X 0.9 / 100 = 225 mg NaCl needed to make the solution isotonic = 225 – 38.75 = 186.25 mg 7. The freezing point depression of a 1% solution of pilocarpine is 0.14. What is the conc. of pilocarpine that will be isotonic with the eye? (Fp depression of normal saline is 0.52) 1% 0.14 x% 0.52 The conc. of pilocarpine that will be isotonic with the eye = 0.52 X 1 / 0.14 = 3.71% XVI- Electrolyte Solution: These are preparations used to treat disturbances in body electrolyte balance. Concentrations of these electrolytes are almost exclusively expressed in terms of milli-equivalent (mEq) which reflects a unit chemical activity. The mEq of any substance represents the amount of a solute equal to 1/1000 of its gram equivalent weight. The equivalent weight = Molecular weight / valency 1. What is the concentration in mg/ml of a solution containing 2 mEq of KCl per ml given that the molecular wt of KCl = 74.5? Equivalent weight of KCl = 74.5 /1 = 74.5 gm 1 mEq KCl = 74.5 / 1000 = 74.5 mg 2 mEq KCl = 74.5 X2 = 149 mg The conc. of the solution = 149 mg / ml 2. What is the concentration of a solution containing 4 mEq/ml of CaCl2 . 2H2O in mg/ml given the molecular wt. of CaCl2 . 2H2O = 147 Equivalent wt. CaCl2 . 2H2O = 147 / 2 = 73.5 gm 1 mEq CaCl2 . 2H2O = 73.5 mg 4 mEq CaCl2 . 2H2O = 4 X 73.5 = 294 mg Conc. of solution = 294 mg / ml 3. A solution is labeled as containing 10 mg % K+. Express this concentration in mEq / L given the molecular weight of K+ is 39. mEq K+ = 39 mg + 10 mg 100 ?? 1000 K conc. = 1000 X 10 / 100 = 100 mg /L = 100 / 39 = 2.56 mEq / L 18 4. A solution contains 15 mg % of Ca++ ions, express this concentration as mEq/L, given the Molecular wt of Ca = 40 mEq Ca++ = 40 / 2 = 20 Ca++ conc. = 15 X 1000 / 100 = 150 mg / L = 150 / 20 = 7.5 mEq / L 5. What is the % conc. of KCl solution containing 20 mEq / 10 ml, given the m wt. of KCl = 74.5? mEq KCl = 74.5 mg 20 mEq = 74.5 X 20 = 1490 mg = 1.49 gm Conc. of solution = 1.49 X 100 / 10 = 14.9 gm % 6. Provided a 1 millimolar soln of a drug (m wt = 200), how much is contained in 50 ml of this soln: a. 0.01 mg c. 0.1 mg b. 1 mg d. 10 mg 7. A Rx required 2 mEq / kg of NaCl for a 70 kg patient. How many ml of saline are needed (M wt of NaCl = 58.5) Amount of NaCl needed = 2 X 70 X 58.5 / 1 X 1000 = 8.19 gm 100 0.9 ???? 8.19 ml of normal saline = 8.19 X 100 / 0.9 = 910 ml XVII- Osmotic Activity: Electrolytes play their part in controlling body water volume through exerting osmotic pressure. This pressure is proportional to the total number of particles in solution. The unit of osmotic activity is called the milli-osmol (mosm). Solutes which dissociate in solution (i.e. electrolytes) exert an osmotic pressure based on the number of particles (ions) present in solution after they have dissociated; e.g. NaCl dissociates to 2 particles (ions) in solution, thus each milli-mole (mmole, m. wt. expressed in mg) of NaCl exerts 2 mosm,. Likewise, CaCl2 dissociates to Ca++ & 2 Cl-, thus each mmole CaCl2 exerts 3 mosm. Solutes which do not dissociate in solution, e.g. glucose, exert 1 mosm for each mmole. m osmol = m moles X no of ions = (wt in mg / m wt) X no of ions 1. A solution containing 5% anhydrous dextrose, how many mosm / L are represented by this conc. given the molecular wt of dextrose = 180? 1 mmole dextrose = 180 mg = 1 mosm 5% dextrose solution = 50 gm / L = 50,000 mg / L = 50,000/180 = 277.78 mosm / L 2. A solution contains 40 mosm / L of K+, how much K+ is contained in each 100 ml of solution, given the m. wt. of K+ = 39? mmole K+ = 39 mg = 1 mosm K+ amount of K+ in 100 ml = 40 X 100 / 1000 = 4 mosm = 4 X 39 = 156 mg / 100 ml 19 3. How many mosm are contained in a solution of normal saline of 1L, (m.wt. of NaCl = 58.5)? mosm of NaCl = 58.5 / 2 = 29.25 mg NaCl in 1 L normal saline = 0.9 X 1000/100 = 9 gm = 9000 mg NaCl = 307.69 mosm / L 4. A physician requires to prepare 1 L of an IV solution containing 0.5 mEq Ca++ / 10 c.c. of solution. How much CaCl2 is needed to make this solution? (M. wt. of CaCl2 = 147) mEq of CaCl2 = 147 / 2 = 73.5 mg CaCl2 needed to prepare 1 L = 0.5 X 1000 / 10 = 50 mEq = 50 X 73.5 = 3675 mg = 3.675 gm XVIII- HLB: 1. Calculate the HLB of the oil phase of the Rx shown to help in selecting a suitable emulsifier. Rx Mineral oil (HLB = 12) 35% Lanoline (HLB = 11) 1% Cetyl alcohol (HLB = 15) 1% O/W emulsifier Water 8% 55% Calculate the HLB of the oil phase to help in selecting a suitable emulsifier. HLB = (Product 1 % X HLB1) + (Product 2 % X HLB2) + … Product 1 % + Product 2 % + ……. HLB of the oil phase = (35 X 12) + (1 X 11) + (1X 15) / 35 + 1 + 1 = 446 / 37 = 12.05 XIX- Displacement: Displacement value is the number of grams of a substance that displace 1 gm of cocoa butter e.g. 1 part of ZnO displace 1 part of cocoa butter. 1. 2g suppositories are to be made with 200 mg ZnO in each, if the displacement value for ZnO is 4, calculate the amount of cocoa butter in each suppository: 4000 mg ZnO will displace 1000 mg cocoa butter 200 mg ZnO will displace X mg cocoa butter, X = 200 X 1000 / 4000 = 50 mg cocoa butter Amount of cocoa butter needed = 2000 – 50 = 1950 mg / suppository 2. Calculate the amount of cocoa butter needed to make 6 suppositories, each containing 300 mg of CuSO4 if each suppository mold holds 2 gm of pure cocoa butter provided that the displacement value for CuSO4 is 2.5? Grams of CuSO4 = 300 X 6 / 1000 = 1.8 gm Amount of cocoa butter displaced = 1.8 / 2.5 = 0.72 Amount of cocoa butter needed = (6 X 2) – 0.72 = 11.28 gm 3. When 10 gm of solute were used to prepare 500 mg/ml solution, the volume of solvent used was 17 ml. Calculate the volume of solvent needed if the 10 gm were used to prepare 1000 mg/ml solution. Volume of 500 mg/ml solution = 10,000 X 1 / 500 = 20 ml solution Thus the volume that 10 gm of solute displaced is = 20 – 17 = 3 ml Volume of 1,000 mg/ml solution = 10,000 X 1 / 1,000 = 10 ml solution But 10 gm of solute displace 3 ml of solvent, so the volume of solute needed = 10 – 3 = 7 ml N.B. When a powdered drug is reconstituted, the volume occupied by the powder must be considered. 20 XX- pH Calculations: 1. Find the pH of a solution that has a H+ conc. of 6 X 10 – 4 : pH = – Log [ H+] pH = – Log ( 6 X 10 – 4 ) = – (Log 6 + Log 10 – 4 ) = – [ 0.7782 + (– 4 ) ] = – [ – 3.2218 ] = 3.2218 2. Calculate the pH when [H+] = 9 X 10 -6 pH = – Log ( 9 X 10 – 6 ) = – (Log 9 + Log 10 – 6 ) = – log 9 + 6 ] = 5.045 3. The pH of a given solution is 11.1, calculate the H+ conc. of the solution : pH = – Log [H+] 11.1 = – Log H+ Log H+ = – 11.1 [ H+] = anti log (– 11.1) = 7.94 X 10 – 12 4. The pH of a given solution is 7.43, calculate the H+ conc. of the solution : 7.43 = – Log H+ Log H+ = – 7.43 [ H+] = anti log (– 7.43) = 3.715 X 10 – 8 5. What is the pH of a 0.1 N NaOH ? pH = pKw – pOH = 14 – pOH pOH = – log [OH] = – log 0.1 = - log 10-1 = 1 pH = 14 – 1 = 13 6. The dissociation constant (Ka) of acetic acid is 1.75 X 10-5. What is its pKa? pKa = - log [Ka] = - log (1.75 X 10-5) = - log 1.75 + 5 = 4.757 7. What is the pH of a buffer prepared from 0.05 M Na borate & 0.005M boric acid (pKa = 9.24). pH = pKa + log [ Salt / Acid ] = 9.42 + log [ 0.05 / 0.005] = 9.42 + log 10 = 9.42 + 1 = 10.42 8. What is the pH of a buffer prepared from 0.005 M NH3 & 0.05 M NH4Cl. (pKb of NH3 = 1.8 X 10-5)? pKb = - log [Kb] = - log [ 1.8 X 10-5 ] = - log 1.8 – log 10-5 = 5 – 0.255 = 4.745 pH = pKw – pKb + log [ Base / Salt ] = 14 – 4.745 + log [ 0.005 / 0.05 ] = 9.255 + log 0.1 = 9.255 + log 10-1 = 9.255 - 1 = 8.255 21 9. What is the molar ratio of the Salt / Acid required to prepare a Na acetate / acetic acid buffer having a pH = 5.76 if the pKa of acetic acid is 4.76? pH = pKa + log [ Salt / Acid ] 5.76 = 4.76 + log [ Salt / Acid ] log [ Salt / Acid ] = 5.76 – 4.76 = 1 Anti-log 1 = 10 Salt / Acid = 10 : 1 10. What is the pH at which 50% of the weak morphine base (pKb = 6.13) will remain in the unionized form? pH = pKa + log [ Base / Salt ] = 14 – 6.13 + log 1 = 14 – 6.13 + 0 = 7.87 A simple solution: 50% of a weak base will be ionized when the pH = pKa Thus, pH = 14 – pKb = 14 – 6.13 = 7.87 11. Calculate the pH of a buffer solution which is 0.1 M with respect to both acetic acid & Na acetate using the Henderson Hasselbach equation, given the pKa of acetic acid = 1.8 X 10-5 pKa = - log [ 1.8 X 10-5] = - log 1.8 + 5 = 5 – 0.255 = 4.745 pH = pKa + log [ Salt / Acid ] = 4.745 + log 1 = 4.745 12. Morphine base has a pKa of 7.83. At what pH will 90% of morphine remain unionized? pH = pKa + log [ Base / Salt ] pH = 7.83 + log 9 = 7.83 + 0.954 = 8.78 13. Morphine base has a pKa of 7.83. At what pH will 90% of morphine be ionized? pH = pKa + log [ 10 / 90 ] pH = 7.83 + log .111 = 7.83 - 0.954 = 6.87 14. Calculate the change in pH upon adding 0.04 mole of NaOH to a liter of a buffer solution of 0.2 M conc. of Na acetate & acetic acid, given the pKa value of acetic acid at 25oC = 4.76. pH = pKa + Log [ Salt / Acid ] = 4.76 + log (0.2 / 0.2) = 4.76 + Log 1 = 4.76 + 0 = 4.76 The addition of 0.04 M NaOH converts 0.04 M of acetic acid to Na acetate conc. of acetic acid is & that of Na acetate is by equal amounts as per the following equation pH = pKa + Log [ (Salt + Base) / (Acid – Base) ] = 4.76 + Log (0.24 / 0.16) = 4.76 + 0.1761 = 4.94, Since the pH before adding NaOH was 4.76 Thus the change in pH = 4.94 - 4.76 = 0.176 22 XXI- Sensitivity Requirements: 1. What is the minimum quantity that can be weighed on a balance with a sensitivity requirement of 6 mg if an error of 5% is permissible: Sensitivity requirement = minimum quantity to be weighed in mg X permissible error S. req. = (x) mg X 5/100 6 mg = (x) mg X 5/100 (x) mg = 6 X 100 / 5 = 120 mg 2. What is the minimum quantity that can be weighed on a balance with a sensitivity requirement of 15 mg if an error of 5% is permissible: 15 mg = (x) mg X 5/100 (x) mg = 15 X 100 / 5 = 300 mg 23 XXII- Differentiation & Integration: A - Differentiation X m = m X m-1 X3=3X2 e 2x = 2 e 2x differentiate the power first (d 2x = 2), place it before the e raised to the same power ln X = 1 / X B – Integration I X m . dx = X m+1 +C m+1 I X 2 + X 4 + X 6 . dx = (X 3 / 3 + X 5 / 5 + X 7 / 7) + C I e 2x e 2x 2 . dx = +C differentiate the power first (d 2x = 2), place it denominator for e raised to the same power + C I 1 / X . dx = ln X + C or I dx / X = ln X + C N.B. Ln e –x = - x ln e Since e & ln cancel each other Thus, Ln e –x = - x ln e = - x 1. If y = 1+2x2 + 3x3 & if x = 1, then dx / dy is equal to: a. 9. Since y = 1+2x2 + 3x3 b. 5. Thus, dx / dy = 4x + 9x2 = 4 + 9 = 13 c. 10. d. 13. 2. If y = 1 + 2 + 3 x x2 x 3 y = x-1 + 2x-2 + 3x-3 dy / dx = -x-2 - 4x-3 - 9x-4 3. Integrate – k . dx where k is constant I – k . dx = kx + C 24 IMPORTANT EQUATIONS 1. Young’s Rule: Child dose = [Age X Adult dose] / (Age + 12) 2. Couling’s Rule: Infant dose = [Age (at next birthday) X Adult dose] / 24 3. Fried’s Rule: Infant dose = [Age (in months) X Adult dose] / 150 4. Clark’s Rule: Child dose = [Weight (in lbs) X Adult dose] / 150 D=M/V Sp. gr. = Wt / V Sp. vol = V / Wt thus sp. volume = 1 / sp. gr Wt. of the liquid = sp. gr. of liquid X volume of the liquid Volume of a liquid = Wt of liquid / sp. gr. Strength X Volume = Strength X Volume 9 X oC = 5 X oF -160 Proof Gallon = Wine gallon X Proof strength / 50% Sodium chloride 0.90% Dextrose 5.00% Boric acid 1.73% Fp depression of normal saline is 0.52 The equivalent weight = Molecular weight / valency m osmol = m moles X no of ions = (wt in mg / m wt) X no of ions HLB = (Product 1 % X HLB1) + (Product 2 % X HLB2) + … Product 1 % + Product 2 % + ……. 25 pH = – Log [ H+] pOH = – log [OH] pH = pKw – pOH = 14 – pOH pKa = - log [Ka] pKb = - log [Kb] pH = pKa + log [ Salt / Acid ] pH = pKa + log [ Base / Salt ] pH = pKw – pKb + log [ Base / Salt ] Sensitivity requirement = minimum quantity to be weighed in mg X permissible error X m = m X m-1 e 2x = 2 e 2x ln X = 1 / X I X m . dx = I e 2x . dx = X m+1 +C m+1 e 2x 2 +C I 1 / X . dx = ln X + C Ln e –x = - x ln e = - x Inventory Turnover = Sales Largest Inventory 26 1. How many ml of a soln of 1:750 are needed to prepare 60 ml of a solution of 1:10,000? 1 : 750 soln = 1 X 100 / 750 = 0.133% 1 : 10,000 soln = 1 X 100 / 10,000 = 0.01% ml of 1 : 750 needed = 0.01 X 60 / 0.133 = 4.51 ml 27







