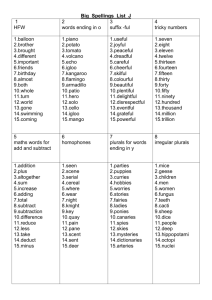
Table 9
advertisement

South Pasadena AP Chemistry Name _________________________________ Period ___ Date ___/___/___ 16 Chemical Equilibrium The ICE box is an organizational tool to keep track of chemicals before, during, and after a chemical reaction. ANSWERS to THE I.C.E. BOX Try these other problems. 1) 2N2O(g) + O2(g) 4NO(g) Consider the reaction: 2N2O(g) + O2(g) 4NO(g) If we started with 1.0 M N2O and 1.0 M O2 only, the reaction might go until we have 2.0 M NO. Follow the steps. Make sure you know what happened in each step. Notice that only the “change” row matches the coefficients in the equation. 2N2O(g) + O2(g) initial 1.0 1.0 initial 2.0 2.0 0 change -1.0 -0.5 +2.0 ending 1.0 1.5 2.0 2) 2N2O(g) + O2(g) 4NO(g) initial 2.0 2.0 0 4NO(g) change -1.0 -0.5 +2.0 0 ending 1.0 1.5 2.0 change ending 2.0 3) N2(g) 2N2O(g) + O2(g) initial 1.0 1.0 4NO(g) 0 change +2.0 ending 2.0 2.0 2.0 0 change -0.5 -1.5 +1.0 ending 1.5 0.5 1.0 4) 4NO(g) initial 1.0 1.0 0 change -1.0 -0.5 +2.0 ending 2NH3(g) initial N2(g) 2N2O(g) + O2(g) + 3H2(g) + 3H2(g) 2NH3(g) initial 1.0 2.0 0 change -0.5 -1.5 +1.0 ending 0.5 0.5 1.0 MgCO3 MgO 2.0 5) 2N2O(g) + O2(g) 4NO(g) + CO2 initial 1.0 1.0 0 initial 2.0 0 0 change -1.0 -0.5 +2.0 change -1.5 +1.5 +1.5 ending 0 0.5 2.0 ending 0.5 1.5 1.5