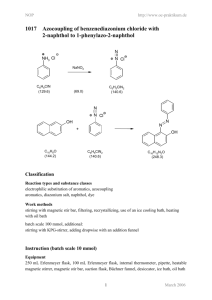
1 Nucleophilic Substitution Reaction: Introduction: The purpose of
advertisement

1 Nucleophilic Substitution Reaction: CH3(CH2)6CH2 KCl or KI, catalyst Br CH3(CH2)6CH2 X 1 hour 60oC Introduction: The purpose of this experiment is to compare the reactivities of a two nucleophiles, iodide and chloride, in a substitution reaction of 1-bromooctane. A quaternary phosphonium salt, hexadecyltributylphosphonium bromide, will be used to catalyze the reaction. nBu CH3(CH2)15 P + nBu Br- nBu hexadecyltributylphosphonium bromide Safety Considerations: Conduct this reaction under the hood. Experimental Procedure: Conduct one of the two following reactions: Iodide: Place 10 mmol of bromooctane and 0.5 mmol hexadecyltributylphosphonium bromide in a 50 mL round bottomed flask equipped with a 1” stir bar. Add 15.7 mL (50 mmol) of saturated KI. Chloride: Place 10 mmol of bromooctane and 0.5 mmol hexadecyltributylphosphonium bromide in a 25 mL round bottomed flask equipped with a 1/2” stir bar. Add 6.5 mL (50 mmol) of saturated KCl solution. Attach a water cooled condensor to your flask (see figure 7.6 in your lab manual). Stir the reaction mixture vigorously and heat it for 1 hour in a 60oC water bath. Use 10 mL of petroleum ether to transfer the reaction mixture to a separatory funnel. Remove the aqueous layer and wash the organic layer with 15 mL of water. Dry the organic layer with anhydrous calcium chloride. Filter the resulting through a pipet containing of 1.5 g of alumina. (Use cotton to plug the bottom of the pipet.) Use air pressure to force the solvent through. 2 Analysis of Results Using Gas Chromatography: Inject 5 microliters of the resulting solution into a gas chromatograph equipped with a non-polar column and thermal conductivity detector. Use the gas chromatography integration data to determine the ratio of starting material to product for your reaction and for that of a classmate who used the other nucleophile. Tabulate your results. Postlab Questions: 1. Compare the results of the KI and KCl reactions. How do the results differ? How can you account for the difference? 2. Predict the results you would have obtained if you had used 2-bromo, 2-methylheptane instead of 1bromooctane. Explain your prediction.