Worksheet 3-2 - colgur chemistry
advertisement

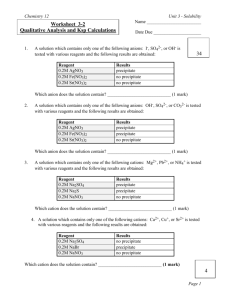
Chemistry 12 Worksheet 3-2 Name ________________________ Date Due _____________________ 34 Do Corrections and Hand In by _____________________ Chemistry 12—Worksheet 3-2 Qualitative Analysis and Ksp Calculations This worksheet covers material from Tutorials 9 and 10 class notes and SW for Unit 3. These questions will be marked and the mark recorded in the “Homework” section in the mark book. One set of corrections will be allowed. 1. A solution which contains only one of the following anions: I-, SO42-, or OH- is tested with various reagents and the following results are obtained: Reagent 0.2M AgNO3 0.2M Fe(NO3)2 0.2M Sr(NO3)2 Results precipitate no precipitate no precipitate Which anion does the solution contain? ____________________________ (1 mark) 2. A solution which contains only one of the following anions: OH-, SO42-, or CO32- is tested with various reagents and the following results are obtained: Reagent 0.2M AgNO3 0.2M Fe(NO3)2 0.2M Sr(NO3)2 Results precipitate precipitate no precipitate Which anion does the solution contain? ____________________________ (1 mark) 3. A solution which contains only one of the following cations: Mg2+, Pb2+, or NH4+ is tested with various reagents and the following results are obtained: Reagent 0.2M Na2SO4 0.2M Na2S 0.2M NaNO3 Results precipitate precipitate no precipitate Which cation does the solution contain? ____________________________ (1 mark) 3 Worksheet 3-2 - Qualitative Analysis and Ksp Calculations Page 1 Chemistry 12 4. Worksheet 3-2 A solution which contains only one of the following cations: Ca2+, Cu+, or Sr2+ is tested with various reagents and the following results are obtained: Reagent 0.2M Na2SO4 0.2M NaBr 0.2M NaNO3 Results no precipitate precipitate no precipitate Which cation does the solution contain? ____________________________ (1 mark) 5. A solution contains Fe3+, Ca2+, Cu+, and Be2+. What compounds (give correct formula for each compound) could be added, and in what order, to separate these ions. Answer this question by filling in the following chart: (8 marks) First compound - _________________________________________ Net-ionic equation for the precipitation reaction: _________________________________ Second compound - _________________________________________ Net-ionic equation for the precipitation reaction: _________________________________ Third compound - _________________________________________ Net-ionic equation for the precipitation reaction: _________________________________ Fourth compound - _________________________________________ Net-ionic equation for the precipitation reaction: _________________________________ 6. A saturated solution of BaF2 has a [F-] of 8.6 x 103-M at a certain temperature. Calculate the Ksp at this temperature. Show all your steps in a logical manner. Correct use of units and significant digits counts (3 marks) Answer _____________________________ 12 Worksheet 3-2 - Qualitative Analysis and Ksp Calculations Page 2 Chemistry 12 7. Worksheet 3-2 Calculate the [Mg2+] in a saturated solution of Mg(OH)2 at 25°C. Show all your steps in a logical manner. Correct use of units and significant digits counts (3 marks) Answer _____________________________ 8. Calculate the mass of AgIO3 which will dissolve in 2.50 L of water at 25°C. Show all your steps in a logical manner. Correct use of units and significant digits counts (3 marks) Answer _____________________________ 9. At a certain temperature 2.2 x 10-4 grams of CuI will dissolve in 1.0 L of water. Calculate the Ksp for CuI at this temperature. Show all your steps in a logical manner. Correct use of units and significant digits counts (3 marks) 9 Answer _____________________________ Worksheet 3-2 - Qualitative Analysis and Ksp Calculations Page 3 Chemistry 12 10. Worksheet 3-2 Calculate the molar solubility of silver chromate in water at 25°C. Show all your steps in a logical manner. Correct use of units and significant digits counts (3 marks) Answer _____________________________ 11. At a certain temperature 0.0558 grams of SrF2 will dissolve in 500.0 mL of water. Calculate the Ksp for SrF2 at this temperature. Show all your steps in a logical manner. Correct use of units and significant digits counts (3 marks) Answer _____________________________ 12. Which is most soluble in water at 25 °C, lead (II) bromide, lead (II) chloride, lead (II) iodide, or lead (II) iodate? (2 marks) Answer ________________. How did you obtain your answer? ____________________ ____________________________________________________________________ 13. Which is least soluble in water at 25 °C, lead (II) bromide, lead (II) chloride, lead (II) iodide, or lead (II) iodate? (2 marks) Answer ________________. How did you obtain your answer? ____________________ ____________________________________________________________________ 10 Worksheet 3-2 - Qualitative Analysis and Ksp Calculations Page 4