NUCLEAR CHEMISTRY UNIT
advertisement

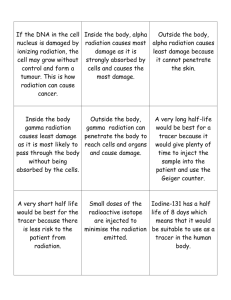
E3 Lesson One Instructor: Cindy Johnson School: Palacios High School Unit Topic: Nuclear Chemistry Lesson Title: The Effects Of Low Dose Radiation on Polymers Subject: Chemistry Grades: 10-12 grades Proposed time frame Unit -2 weeks Project lesson - 1week Beginning of October Overview of the Lesson: To learn about radiation, the types of radiation emitted and their properties, and the affects of radiation on matter Integration of the E3 RET experience in the lesson will be through a lab activity and class discussion Educational Correlations: TAKS 1 TEKS 1;2;6B;9A,D;11B,C Class Objectives: The student will: Identify alpha, beta, and gamma radiation in terms of composition and properties Explain radioactivity, nuclear stability, radioisotopes, decay Describe methods to detect and measure radiation including units Describe the effects of radiation on matter and tissues Lesson engagement activities: Independent activities Peer tutoring Parings in groups of 2-4 Hands-on activities Whole group instruction Technology integration Lecture Materials/ Supplies Geiger counters Radiation sources such as smoke detectors, mantles, etc. Polymers- CR-39, lexan, acetate, plastic cover slips Sodium Hydroxide Microscopes Hot plate/ stirrer Wire and alligator clips or clothes pins Glass Container such as a large culture dish Pieces of concrete or lead Storage dish- Petri dish Other resources: textbook web- computer Videos clips from Region 3 Lesson One Outline: Pretest over the material: Define radioactivity, decay, nuclear stability, radioisotopes Discuss stable and unstable isotopes. Describe the three major types of radiation- alpha, beta, and gamma rays Explain to students what a Geiger counter is and how it works. Activity One The students will use the Geiger counter to examine different materials for radioactivity and record the item and the amount of radiation detected for that item on a chart. The students will then analyze, rank, and discuss their results Activity Two (E3 integration) Divide the students into groups. The source of radiation, Americium-241, is removed from a smoke detector or an alpha source is purchased from a scientific catalogue. The polymers (CR-39, polycarbonate, plastic slides, and acetate) are cut into small squares, which the teacher will do before lab. A CD or memory card from a cell phone or camera will also be used to make applicable to everyday life. The student will determine the amount of radiation emitted from the source using a Geiger counter. The students will expose the polymers to the radiation source for a given period of time by placing the source on the polymer. The length of time depends upon the strength of the source and the polymer. The Americium from the smoke detector only needs about 1 minute on the CR-39, longer on the Lexan. The students will remove the source, label the exposed side, and place them in a labeled plastic Petri dish. The students will also expose a CD or memory card to radiation for several days. The teacher will place the exposed polymers in a 6.5N NaOH solution for 3-4 hours to etch them. Lexan takes 4-5 hours. The teacher will wash the polymers under running water and air dry them. The students will then examine the polymers using a microscope. They should be able to see the penetrations or tracks formed by the radiation. The students will count the holes for measurement of alpha particle penetrations using a digital microscope and take pictures of their polymers and count the penetrations from the picture in a prescribed area. This could be very difficult to do on the Cr-39 if there are a large number of holes. One might put a small dot on the back side of the CR-39 in the crowded area and one in the less dense area and count the penetrations in each mark and average them together. The students will estimate the number of penetrations in each square. They will rank the polymers as to their sensitivity to radiation and determine which material would be the best at detecting radiation. The students will determine which polymer would be the most cost effective in making a radiation detector. The students will play the CDs or memory cards to determine how susceptible the CDs or SD cards are to low doses of radiation. They will determine which media storage device would be the best or would corrupt the fastest. Activity Three Divide the students into groups. Have the students devise an experiment to determine the best materials, from a list, to use to effectively stop alpha and beta radiation. The students will then run their experiment using the radiation sources given to them and their chosen materials and a Geiger counter to collect their data. They will then analyze their data and draw a conclusion. They will be asked to justify their answer. Discuss radioactive isotopes use in medicine and research and how radiation can damage tissue Using the internet, research the types of radiation or radioisotopes Post test over unit (See next page) Pre/Post Test over Nuclear Chemistry Lesson One _____ 1. Which type of radiation is composed of a Helium nucleus? a. alpha b. beta c. gamma d. positron _____ 2. Radioactive atoms throw off particles and/or rays in a process called a. half-life b. decay c. fission d. fusion _____ 3. An instrument that is used to detect and measure radiation is a a. microscope b. telescope c. seismograph d. Geiger counter _____4. What kind of energy is released by unstable isotopes? a. electricity b. static c. potential d. radiation _____ 5. Which type of radiation is not a particle, but a type of electromagnetic wave? a. alpha b. beta c. gamma d. positron _____6. The effect of radiation on people is measured in a. millirem b. rad c. Gray d. electron volts _____ 7. Natural background radiation occurs from a. radon b. cosmic raysc. rocks/soil d. all of these _____ 8. Which type of radiation has a negative charge? a. alpha b. beta c. gamma d. positron _____ 9. What material is made up of repeating units called monomers? a. radiation b. matter c. polymers d. isotopes _____10. Alpha radiation is shielded or blocked by which material listed below? a. paper b. skin c. concrete d. aluminum e. all of these f. none of these _____11. Gamma radiation is shielded by which material listed below? a. paper b. skin c. concrete d. aluminum e. all of these f. none of these _____12. A form of an element that naturally gives off radiation is called a/an a. radioisotope b. isotope c. radioactive element d. half-life _____13. The process by which materials give off particles/rays is called a. radiation b. radioisotopes c. radioactivity d. stability _____14. Nuclear stability depends upon what? a. the proton to electron ratio b. the electron to neutron ration c. a 1:1 proton ration d. the proton to neutron ratio _____15. The natural occurring radiation in the environment is called a. e-voltage b. background radiation c. nucleon d. ionizing radiation _____16. The unit that measures the absorbed dose of radiation is called a a. rem b. millirem c. rad d. e volt