Standard Enthalpies of Formation (ΔH°f)
advertisement

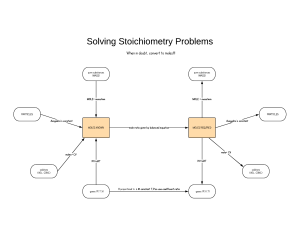
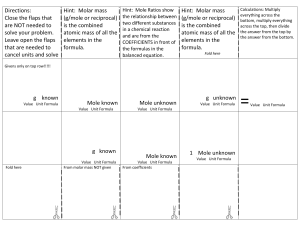
Standard Enthalpies of Formation (ΔH°f) quantities of energy associated with the formation of 1 mole of a substance from its elements in their standard states (SATP- 25°C and 100kPa) *The units for ΔH°f are always kJ/mol because the values are always given for 1 mole Writing formation equations: 1. Write one mole of product in the state indicated in the question 2. Write the reactant ELEMENTS in their standard states (as seen on the periodic table) 3. Balance the equation to yield one mole of the product Example: Write the equation for the formation of liquid ethanol Now you try: Write an equation for the formation of solid calcium carbonate Using ΔH°f to find ΔH (examples) What is the thermochemical equation for the reaction of lime (calcium oxide) and water. Use ΔH°f values to calculate. (p.s. you can find these on p799) Example: #2 What is the MOLAR enthalpy of combustion of methane fuel? (a gas)