Advanced Chemistry Titration Lab
advertisement

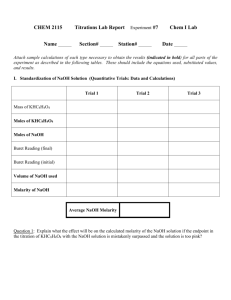
Advanced Chemistry Titration Lab Your objective in this lab is to evaluate the cost effectiveness of four brands of antacid tablets. You will design, perform, and document the procedure and analyze and present the results. Antacids neutralize (or buffer) the excess hydronium ion, H30+, (H+) in stomach acid to relieve this discomfort. The amount of antacid needed for relief is dependent upon its “strength” which is based on its ingredients. The formulations of some common antacids are listed below. Common Antacids Principal Active Ingredient(s) NaHCO3/citric acid Mg(OH)2/Al(OH)3 CaCO3/Mg(OH)2 CaCO3 Al(OH)3/MgCO3 Al(OH)3/MgCO3/Mg(OH)2 Representative Antacid Alka Seltzer Mylanta Rolaids Tums, Equate Gaviscon Di-Gel The advertised antacids that buffer excess acid in the stomach are those containing calcium carbonate, CaCO3, or sodium bicarbonate, NaHCO3. These antacids establish the HCO3-/CO32- buffer system in the stomach. CO32-(aq) + 2H30+(aq) HCO3-(aq) + 2H2O(l) Antacids, like Rolaids, that also contain Mg(OH)2 or Al(OH)3 are a combination antacid that also reacts with stomach acid. Mg(OH)2(aq) + CaCO3(aq) + 3H3O+(aq) Mg2+(aq) + Ca2+(aq) + 5H2O(l) + HCO3-(aq) The antacids you will use are as follows: ____________________________________________________ Price Quantity Dosage (# tablets) (# tablets) Rolaids $ 150 2-4 Equate $ 150 2-4 Tums $ 150 2-4 Alka Seltzer $ 36 2 Mylanta $ 355mL 2-4 teaspoons (1 tsp = 5mL) ____________________________________________________ You will need to make a standard sodium hydroxide solution. You will also need to make a standard hydrochloric acid solution of about 0.1M (since that’s about what your NaOH standard is). I will provide 3M HCL for you to dilute. I recommend you titrate your final HCl solution to check your dilution. As for indicators there is phenolphthalien and bromophenol blue. Because you will be dealing with substances that form buffer solutions, you will be using a "back titration" process to evaluate effectiveness of the antacid. This process is described below. Determine a way to evaluate the effectiveness of each antacid and relate the effectiveness to cost. Document your procedure, data and results in the form of a typed memo to me. Show all your calculations in a neatly handwritten attachment to the memo. Procedure for Standard NaOH solution: You are to complete at least three trials in the standardization of your NaOH solution. To hasten the analysis, clean, dry and label three 125 mL or 250 mL Erlenmeyer flasks and measure the mass of three KHC8H4O4 samples (see below). If all the balances are occupied, prepare your NaOH solution first. This is an analytical experiment; you are striving for “good” results. The measured molar concentration of the NaOH for the three trials should be within ±1%. If not, a fourth or fifth trial may be necessary. Preparation of NaOH solution Using a 10-mL pipet, carefully transfer 10 mL of the concentrated NaOH solution to a 500-mL polyethylene bottle. (Caution: a concentrated NaOH solution causes severe burns) Dilute to approximately 500 mL with previously boiled, distilled water. Boiling the water removes traces of CO2. Stir the solution for several minutes (Do not shake the bottle; this increases the probability of CO2 absorption.) Cap the polyethylene bottle to prevent absorption of CO2 by the NaOH solution. Label the bottle. Preparation of KHC8H4O4 samples Measure 0.3 – 0.5g (±0.001g) of the dry KHC8H4O4 and place in a clean, dry 125-mL or 250-mL Erlenmeyer flask. Add 50 mL of distilled water and 3 drops of phenolphthalein. Determination of NaOH solution Concentration Prepare a buret for use with the NaOH solution as the titrant. Record the starting level of the buret. Titrate the KHC8H4O4 sample to its endpoint. Record the ending level of the buret. Calculate the concentration of the NaOH solution using the mass of KHC8H4O4 and the volume of NaOH solution used in the titration. After completing titrations of all three samples, compare the molarities calculated for each sample. If the molarities are within ±1% of each other, average them for the final molarity of your standard NaOH solution. Label the polyethylene bottle with the averaged molarity. Back Titration In back titration, what we are essentially doing is reacting a known amount of the antacid with a known amount of acid. We continue doing this until the antacid will no longer neutralize the acid (ie., there is excess acid in the flask). Then we titrate the acid in the flask to determine its quantity, thus determining the quantity of acid that the antacid neutralized. Basically: Since an antacid has the same neutralizing effect on stomach acid as does NaOH, the amount of antacid in a sample is called its NaOHequivalent. To determine the NaOHequivalent for an antacid, we subtract the excess moles of HCl(aq) from the total moles of HCl(aq) added to the antacid. NaOHequivalent = HCl(aq)total – HCl(aq)excess General Procedure: 1. Add 25.0 mL of standardized HCl into the flask containing the antacid and swirl to dissolve the antacid. 2. Slowly heat the solution to boiling and continue to heat at a gentle boil, for at least 1 minute, to expel dissolved CO2. Add 4-8 drops of bromophenol blue indicator. If the solution if blue, add an additional 15.0 mL of HCl and boil again. Continue adding HCl until the solution turns yellow. (Record the total amount of HCl added.) 3. Titrate the solution to the blue endpoint of the bromophenol blue indicator to determine the amount of excess HCl. Rubric for Antacid Analysis Lab NAMES: MEMO: Procedure Documented Data Provided Effectiveness related to cost Methodology appropriate Conclusion provided/supported by data CALCULATIONS Organization Completeness Units Methodology/results supported by calcs RAW SCORE (18 points possible) Raw Score x 5 = TOTAL SCORE