chem 324 syllabus-2013_rc
advertisement

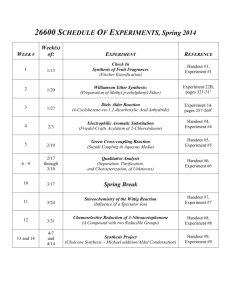
MANHATTAN COLLEGE DEPARTMENT OF CHEMISTRY & BIOCHEMISTRY ORGANIC CHEMISTRY LABORATORY CHEM 324 Spring 2013 SYLLABUS Raymond Chung, PhD Office: Hayden 402 Phones: (718)-862-7211 Office Hours: TBA Laboratory: Hayden 503 Prelab: Hayden 201 Time: Tuesday, Sec. 4, 1:25-5:25 PM Requirements: 1. Students must have successfully completed or be taking Chem 320 concurrently. 2. Students must checkout properly on the specified date or before withdrawing from the course. 3. Students must follow all Safety Regulations while working in the laboratory. 4. Students must obtain their own safety goggles and wear them at all times in the lab. 5. Students must attend pre-lab lectures. Students failing to come to pre-lab will be asked to leave and will be given a zero for the experiment. 6. Students must come prepared to the lab with a written outline of the experimental procedure (prelab.) in the lab notebook. Students who do not come prepared will be asked to leave and will be given zero for that experiment. Coming unprepared is a safety hazard. 7. Students must attend the lab session that they are registered for. Exceptions will only be made in severe (documented) cases. Required Material: Experimental Organic Chemistry, Daniel R. Palleros, John-Wiley, 2000. A ruler, calculator and safety glasses. Course Goals: Purification/Isolation/Synthesis Methods and Criteria of Purity To learn the basic skills required to work in an organic chemistry laboratory, e.g., Reaction setup, chemical isolation & purification, analysis of purity of organic compounds and methods for characterization of compounds by chemical and spectroscopic methods. Course Schedule: (Experiment 1)(Week 1 & 2) Spectroscopy- Identification of unknown organic compounds using infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy. See handout for experimental procedure. (Full Lab Report) (Experiment 2) (Week 2 & 3) Electrophilic Aromatic Substitution– Synthesis of 4-(phydroxyphenyl)-2-butanone (Raspberry Ketone) via Friedel-Crafts Alkylation of phenol with 4-hydroxy-2-butanone. See handout for experimental procedure. (question sheet) (Experiment 3) (Week 4) Grignard Reaction – Synthesis of Malachite Green or Crystal Violet ( triarylmethane dyes) using a Grignard reaction. See handout for experimental procedure. (question sheet) (Experiment 4) (Week 5) Oxidation and Reduction– oxidation of p-toluic acid to terephthalic acid. Reduction of Camphor (a chiral ketone) into borneol and isoborneol using sodium borohydride as a reducing reagent. Experiment E21.2 and E21.7 Read Palleros pgs 438-472.(question sheet) (Experiment 5) (Week 6) Ether Synthesis – Synthesis of 2-methoxynapthelene from methanol and 2-napthol using an acid catalyzed condensation reaction. See handout for experimental procedure.(question sheet) (Experiment 6) (Week 7) Wittig Type Reaction – Preparation of ethyl cinnamate (a fragrance compound found in cinnamon and certain fruit) from benzaldehyde via the use of a stabilized Wittig reagent. See Handout for experimental procedure.(Full Lab Report) (Experiment 7) (Week 8) Esters Formation and Hydrolysis - Preparation of propyl acetate and isoamyl acetate by Fisher esterification . In addition the hydrolysis of the esters in fat (saponification) will be examined. Experiment 22A.2 Palleros 480-485. Also see handout for saponification. (question sheet) (Experiment 8) (Week 9&10) Aldol Reaction and UV-Visible Spectroscopy . - Synthesis of -ionone and/or -ionone (compounds used in the fragrance industry) by the use of an aldol reaction. Experiment 23B Palleros 520-531.(Full Lab Report) (Experiment 9) (Week 11 & 12) Multi-step Synthesis – Synthesis of Lidocaine. The local anesthetic lidocaine (xylocaine®) is synthesize in a 3 step procedure starting from 2,6 – dimethyl-nitrobenzene. The first step involves the reduction of the nitro compound into the corresponding amine. The second step involves amide formation by reacting the amine with an acid chloride. Finally the amine group is introduced in the final step via an Sn2 reaction. See handout for experimental procedure.(Full Lab Report) (Experiment 10) (Week 13) Formation of Azo dyes – a variety of azo dyes will be formed by reacting aromatic diazonium salts with activated aromatic rings (via an electrophilic aromatic substitution reaction). Experiment 27.1 (orange II) & 27.3 Read pages 612-615 and 630-631 in Palleros See Handout for additional information.(question sheet) Final Exam May 9-15 –NOTE: organic lab has a common final which can be scheduled any where during finals week. Do not schedule travel plans during finals week. Grading & Evaluation: A students grade is determined by a combination of their laboratory technique, laboratory reports and tests. Each different criteria is given the following weight. Technique Laboratory Reports Midterm (10 %) and Final Exam (15%) 35% 40% 25% (a) Technique - The technique score is effected by the following: prelab notebook, following proper laboratory safety procedures, lab experiment results, glassware breakage, cleanliness, turning off and returning equipment etc.. A bound composition notebook must be used as the laboratory notebook. This notebook is to contain all necessary pre-lab material as well as a record of all the observations and data collected in the lab in a well organized fashion. (b) Laboratory Reports – A formal report is required for every experiment. The reports must follow the format specified in class. All Laboratory reports must be typed on a word processor (including tables). All chemical structures however may be drawn in by hand. Each laboratory report is due on the dates listed above. Laboratory reports must be handed in on time. Reports that are late suffer a penalty of 5% a day. If a report is not submitted by the time the reports are returned to the class the student receives a zero for the experiment. Most lab reports are graded on the basis of ten points except for a few of the longer Reports must be handed in during class or in my mailbox. DO NOT Slide them under office doors, email them, leave them in the baskets in the hallway or leave them in other locations. multistep experiments which are graded out of 15 or 20 points. (c) Exams - There are two exams in this class, a midterm and final. The date of the midterm is listed in the syllabus and lab calendar. The final is a common exam. Common exams can be scheduled any where during finals week. Do not schedule travel plans during finals week. There are NO make-up exams given under any circumstances. The grades are determined by using the distribution of students overall combined grades. In general, the median grade will be a “C to C+” grade. An “A” grade is given only for distinctive and outstanding achievement. A”B” grade is an above average grade. Attendance: Absence from lab will result in the student getting a zero for that experiment (In both report and technique scores). Missing a test will result in a grade of zero for that test. Exceptions will only be made in severe (documented) cases. College Policies – (1) All written work must conform to standard English usage. Failure to meet such standards will affect your grade. (2) The college subscribes to the principle of academic integrity. Incidents of cheating, facilitation of dishonesty or plagiarism may result in a “F” grade for the course. Copying sections of a lab report from other students is not acceptable. If this occurs all students involved will be penalized. The penalty will be dependent on the severity of the offense. Copying sections of a text book or web site directly into your lab report is also not acceptable. We already know the text book author understands the experiment; the goal of writing the report is to show that you understand it. As with copying from other students this will incur a penalty to your lab grade which depends on the severity of the offense.