BRANDEIS UNIVERSITY
advertisement

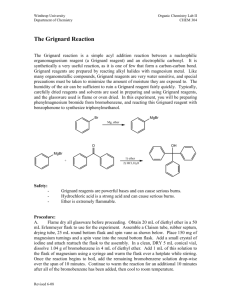
BRANDEIS UNIVERSITY CHEMISTRY 29A PRELAB WRITEUP AND EXPERIMENTAL REPORT SHEET NAME Richard Fan______________ T.A.'S NAME David Gay __ ___ ___________ DATE 7/13/06 _______EXPERIMENT# __1___TITLE _Preparation of a Grignard Reagent___________ PURPOSE _To prepare triphenylmethanol from phenylmagnesium bromide and benzophenone____________ PRELIMINARY WRITEUP: Materials: reaction tubes / caps syringe. Stirring rod, pipettes 50mg Mg shavings 0.7mL, 0.5mL, 1.0mL anhydrous diethyl ether 330mg bromobenzene 0.364g benzophenone 2mL HCl 1mL NaCl 0.5mL dry ether Bromobenzene MW 157.02 BP 156ºC Phenylmagnesium Bromide MW 181.33 % yield = (experimental/theoretical) × 100% Expected weightx = # of moles used × MWx Benzophenone MW 182.22 MP 48ºC Triphenylmethanol MW 260.34 MP 164ºC EXPERIMENTAL PROCEDURES* (Include in preliminary writeup.) MODIFICATIONS AND OBSERVATIONS* (Obtained from the lab experience) -Add to dry tube 50mg Mg shavings, 0.5mL anhydrous diethyl ether, cap the reaction tube -To dry vial, mix 0.22mL bromobenzene, 0.7mL anhydrous diethyl ether -Add 0.1mL of vial contents with syringe to tube, mix by flicking -If no reaction (not cloudy or brown, no bubbles), crush Mg with stirring rod until bubbling -Recap tube, add syringe contents slowly with syringe tip in place, keeping steady bubbling -When done, add stirring bar to tube -To new vial, mix 0.364g benzophenone and 1mL dry ether -Add this to tube slowly while stirring, wait for red to disappear -Add 2mL 3M HCl while on ice, stir -Add ether until all product dissolves, remove water if necessary -Wash with 1mL NaCl -Remove ether layer, dry it using two pipette method (cotton / Na2SO4) -Aspirate ether, weigh crude product, get melting point -Recrystallize with 2-propanol, get final weight, get final melting point 1 Due to the size of the Mg shavings, the approximate amount added was 50.7mg. The initial mixture of Mg, anhydrous diethyl ether, and bromobenzene produced no reaction. Mixture started boiling after several minutes of crushing with a stir rod. After adding the benzophenone and dry ether mix, the product was left alone for several minutes while HCl was being prepared. During this time, a pink solid collected on the bottom of the reaction tube. This was broken up with a spatula as HCl was slowly added. After drying, the initial crude crystal product appeared slightly yellow and weighed 0.4181g. This crude product was dissolved in approximately 2mL of 2-propanol over heat, and then recrystallized over ice. After drying with the Hirsch funnel, the final product was a white crystal weighing 0.1815g. This change in color indicates that some impurity had been removed in the recrystallization process. The melting points (MP’s) for the crude and final products also show that different substances were present. The MP for the crude product was 131ºC-142ºC, which both had a very wide range and was much lower than the expected MP for triphenylmethanol (164ºC). The final MP was much closer to the expected at 161ºC-162ºC, also suggesting that the final product was a more purified form of triphenylmethanol. RESULTS, DISCUSSION AND CONCLUSIONS* The two portions of this experiment were to first prepare the necessary Grignard reagent and then synthesize triphenylmethanol. In the first portion of the experiment, magnesium reacts with bromobenzene in ether to produce phenylmagnesium bromide. Since 2mmol of Mg and about 2mmol of bromobenzene are used for this first reaction, about 2mmol of phenylmagnesium bromide is expected to be the result before beginning the second portion of the experiment. 2mmol of benzophenone is then added to the Grignard reagent. The briefly visible red color may be an indication of the MgBr being transferred from the reagent to the benzophenone to form the intermediate. As this color dissipates, there should be no more free MgBr as they have all bonded to benzophenone. Finally, HCl in water replaced MgBr on the intermediate with a hydrogen ion to complete the alcohol group to form triphenylmethanol. The amount of each of the reagents used in the experiment has been 2mmol, so there should be 2mmol of product – 0.5207g of triphenylmethanol (0.002mol × MW triphenylmethanol 260.34 = 0.5207). The weight of the crude product at this point is 0.4181g, about an 80% yield. The melting point of this crude product was observed to be 131ºC to 142ºC, which has a very wide range and is much lower than the expected melting point of 164ºC. This indicated that the crude product probably had some impurities in it. After dissolving the crude product in about 2mL of 2-propanol and recrystallizing, the final product weighed 0.1815g, about a 35% yield. This final yield was obtained from two crops; after the first drying by Hirsch funnel, some triphenylmethanol crystals were observed in the 2-propanol in the Hirsch funnel catch flask. This mixture was poured through the funnel again and the second crop yield added to the first. The melting point of the final product (obtained from the first crop) was observed to be 162ºC, which is very close to the expected value of 164ºC. This suggests that many of the impurities that existed in the crude product were removed through the recrystallization. There was also a difference in color between the final product and the crude product, which also suggests that some impurity had been removed. Care was taken to use dry equipment for the reaction portions of this experiment, since presence of water would cause byproducts to be formed with phenylmagnesium bromide. The crude product had a relatively high percentage yield, but it may have been due to foreign compounds or moisture. The final percentage yield dropped to less than half of that of the crude product after recrystallization, possibly due to removal of the foreign compounds, but also possibly due to product remaining in solution in 2propanol. The observation of crystals in the catch flask indicated that the product was still recrystallizing as the drying was being carried out. This final mixture should be allowed to recrystallize for a longer period of time. ANSWERS TO END-OF-CHAPTER QUESTIONS* 1. Ethyl benzoate + phenylmagnesium bromide Diethylcarbonate + phenylmagnesium bromide 2. Phenylmagnesium bromide + water 3. Free MgBr may separate, allowing Br to bind to intermediate carbocations. This would leave free Mg in the product mixture. 4. Solid triphenylmethanol product can be dissolved into solution to allow the solid Mg to be filtered out of the solution. The product can then be recrystallized. 2