APES Energy Calculations Worksheet: Practice Problems
advertisement

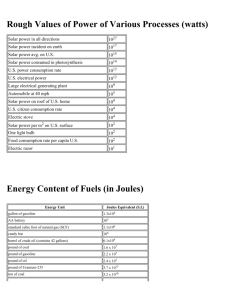
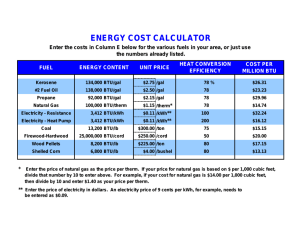
APES: Energy Calculations _________________/_____/_____ Name bell date Energy Vocabulary: Energy: ability to do work---Work is Force times Distance Newton’s 2nd law tells us that force is mass times acceleration So we can use the following units to describe f, m, a: System SI English Force = Newton Lb Mass x Kg Slug (no kidding!) Energy = Joule Ft-lb Force x Newton Lb Acceleration m/s2 Ft/s2 And for energy, we have System SI English Distance Meter ft Newton: SI Unit of force 1 N = 1kg-m/s2 (as you can see from the table above) 1 N = 0.225 lbs. Joule: a fairly small amount of energy, but used often in scientific work. Abbreviation is J (capital letter). (one donut has about 106 joules of energy) Calorie: amount of heat needed to raise the temperature of one gram water by one degree Celsius. 1 calorie = 4.186 joule 1 Kilocalorie = 1000 calories (food calories are Kilocalories) Btu (British Thermal Unit): amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit. One Btu is about the amount of heat released in burning one kitchen match. 1 Btu = 252 cal = 1055 joule Therm: Gas companies measure sales in Therms (thermal units). 1 Therm = 100,000 Btu Natural gas has a heat value of 1030Btu/ft 3, so 105Btu 1therm 1030Btu/ 97.1ft 3 (round to 1 therm = 100ft3) ft 3 NOTE: gas companies don’t use true SI notation, they write 1ccf = 100 ft3 and 1mcf = 1000 ft3 Power: “time rate of doing work”—measured in joules/second or WATTs (W) 1 watt = 1 joule/second Electricity is measured in terms of kilowatt-hours (kWh) by the electric company 1kWh = 1000J/sec x 3600 s = 3.6x10 6 Joule (3600 sec = 1 hour) One kWh is the energy required to power ten 100-watt light bulbs for one hour. Ave. US home uses about 10,000 kWh electric energy/year. Electric Power Plants: Rated in terms of their capacity to deliver electric power. For instance, a large coal-fired or nuclear plant might be rated at 1000 MWe (megawatts—the “e” is for electric and shows that the output energy is in the form of electricity). The input energy is usually measured in terms of the heating value of the fuel—Btu’s for coal or therms for natural gas. If a coal plant operates at 40% efficiency, then energy input required for the plant can be computed as follows: = Input Output 40% 1000MW 0.4 = 2500MW 2500x106 J / sec x 3600sec/ hr 1054 j / Btu = 8.54 x 109 Btu/hr. If this energy is supplied by coal with a heating value of 25 x 106 Btu/ton, then coal would need to be input at a rate of 8.54 x 10 9 Btu/ hr 25 x 106 Btu/ ton = 342 tons/hour Operating at full capacity 24 hours per day, such a plant would consume about 3 million tons of coal per year. Solar Energy: The sun, of course, provides radiant energy for all life on earth, and the rate at which this energy is received is referred to as solar flux, representing the power per unit are received at a given location. At the position of the Earth’s orbit, this number is about 1400W/m2, and is referred to as the solar constant. This means that a flat panel of 1 m2 placed outside the Earth’s atmosphere and oriented perpendicular to the sun’s rays would receive 1400 J/sec of solar energy. Since the atmosphere absorbs about half of this energy, about 700 W/m2 is about the maximum amount that reaches the Earth on a hot summer day in the tropics. At other locations on the Earth’s surface, the solar flux is reduced even further. For example, Tucson, AZ enjoys an annual average solar flux of 250 W/m2, but Cleveland , OH receives only about 160 W/m2. Obviously, such number has implications for the merits of solar heating and cooling as well as biomass growth in various locales. 1. 2. 3. 4. 5. 6. 7. 8. 9. Given that 1 kcal of heat is required to increase the temperature of 1 kg of water by 1°C: a. How many kcals would be required to heat 100 kg of water by 20°C for a bath? b. How many joules is this? (Convert to megajoule for easier number sense) c. How many Btus? d. If your water heater can supply 40 kBtu/h, how long will it take to heat this water? (convert answer to minutes) a. Given that 1 kWh = 3.6 MJ and that 1 Btu = 1055 J, show that 1 kWh = 3412 Btu. b. Why would it be incorrect to use this conversion factor directly to determine the amount of coal required to generate electricity in a power plant? A typical home in the northern U.S. might require 120 MBtu of heat for the average winter. a. If this heat were supplied by a natural gas furnace operating at 60 percent efficiency, 1) How many BTU is needed? 2) How many cubic feet of gas would need to be purchased? You will need to convert ft3 into ccf, the normal unit for natural gas.) b. At a cost of $0.90/ccf, what would it cost to heat this house for one season? c. If a new 80 percent efficient furnace could be installed at a cost of $4,000 1) How many Btu for the new furnace? 2) How many ccf? 3) What would it cost to heat this house for one season with the new furnace? 4) How long would it take to pay back the cost of this furnace assuming gas prices remained the same? Suppose the house in question 3 is located in Cleveland where the annual average solar flux is 160 W/m22. If 10 m2 of solar panels operating at 20 percent efficiency were installed on this house to collect and store solar energy in the form of hot water: a. How much energy could be gained in one year in this manner? (HINT: 8760 hr/year, calculate kWh and then convert to Btu) b. What fraction of the annual heating requirement is this? c. Using the hot-water heating requirements for a bath from question 1(c), how many hot baths would this energy supply in one year? The annual average solar flux in Tucson is 250 W/m2. Suppose 10 m2 of solar electric panels operating at 10 percent efficiency were installed on a home there. a. How many kilowatts would be collected? b. How many kWh of electricity would these panels collect in one year? c. What fraction of the annual electrical requirement of 10,000 kWh for the average home does this represent? Solar energy is converted naturally into wood biomass with an efficiency of about 0.1 percent. Suppose a wood lot of 100 hectares (106 m2) is located in Missouri, where the average annual solar flux is 200 watts/m 2. Given that the heat value for wood is 12 MBtu/ton, a. The wood is equivalent to how many kilowatts? b. How many kilowatt-hours for the year? c. How many BTU’s? d. How many tons of wood can be produced by this property each year? e. Discuss some of the advantages and disadvantages to providing electrical power by each method. Batteries are usually rated in terms of ampere-hours, indicating the current that the cell is capable of delivering for a specified time. Typical D-cell flashlight batteries might be rated at 3 ampere-hours. The total electrical energy available from such a battery is found by multiplying the ampere-hour rating by the battery voltage. Thus this same 1.5 volt D cell could deliver 4.5 watt-hours of electrical energy. a. Convert this energy to kWh and compare the cost of electrical energy derived in this manner to that of standard "grid-based" electricity. Assume that the battery costs $1.00 and that electricity from the power company is available at $0.10/kWh. With moderate winds, a modern large wind turbine can generate about 250 kW of electricity, whereas a large nuclear power plant can generate 1,000 MW. a. How many wind turbines would be required to give the same output as one nuclear power plant? table below gives prices and heat energy content for various fuels that are commonly used for home heating. Fuel prices are given as a perunit cost for fuel delivered to the home. Complete the table by filling in the last two columns and then compare the cost of home heating by these various methods. In your computations, assume that the home requires 120 MBtu of heat for a season and that gas- or oil-fired furnaces operate at 80 percent efficiency. Assume that electrical heating is 100 percent efficient. Fuel Price Energy Content of Fuel Cost per MBtu Cost of Home Heating Nat. gas $1.14/ccf 1030 Btu/cf Fuel oil $1.93/gal 133 k Btu/gal Electricity $0.10/kWh 3412 Btu/kWh 10. The conventional gasoline-powered 2004 Honda Civic is one of the best gas-powered cars in its class for mileage. The conventional Honda Civic gets 35 MPG during city driving (40MPG Highway). When the exact same car is given a hybrid-electric engine, mileage is 47MPG city and 48 MPG highway. $20,650 is the cost of the Civic Hybrid; $16,000 is the cost of the conventional Civic, depending on the features. You plan to drive mostly in the city, to and from work and for weekend errands. You expect to drive 8000 miles a year in the city and 4000 highway miles. a. b. c. How much would you spend on gas for the hybrid Civic in a year, assuming $1.89/gallon? (average price for MidAtlantic states 2/19/08) How much would you spend on gas for the conventional Civic, assuming $3.00/gallon? How long would it take for the savings in gas costs to offset the increase in the price of the hybrid Civic? 11.The 2004 Toyota Prius gets 60 MPG city and 51 MPG highway. The “best-selling car in America” is the Ford F-150 truck. The 4WD, V6 version of the F-150 gets 15 MPG city and 19 MPG highway. A. The average American drives 12,000 miles per year. Assume that most people do about 75% city and 25% highway driving. How many gallons of gas would be saved per person by driving a Prius over an F-150? B. Assume that every gallon of gas burned contributes approximately 20 kg of CO 2 to the atmosphere. How many extra kg of CO2 are put into the atmosphere by the F-150 in a year when compared to the Prius?