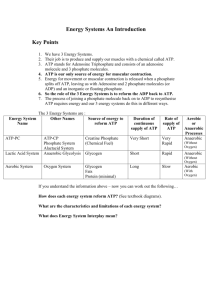
ATP and Energy Notes
advertisement
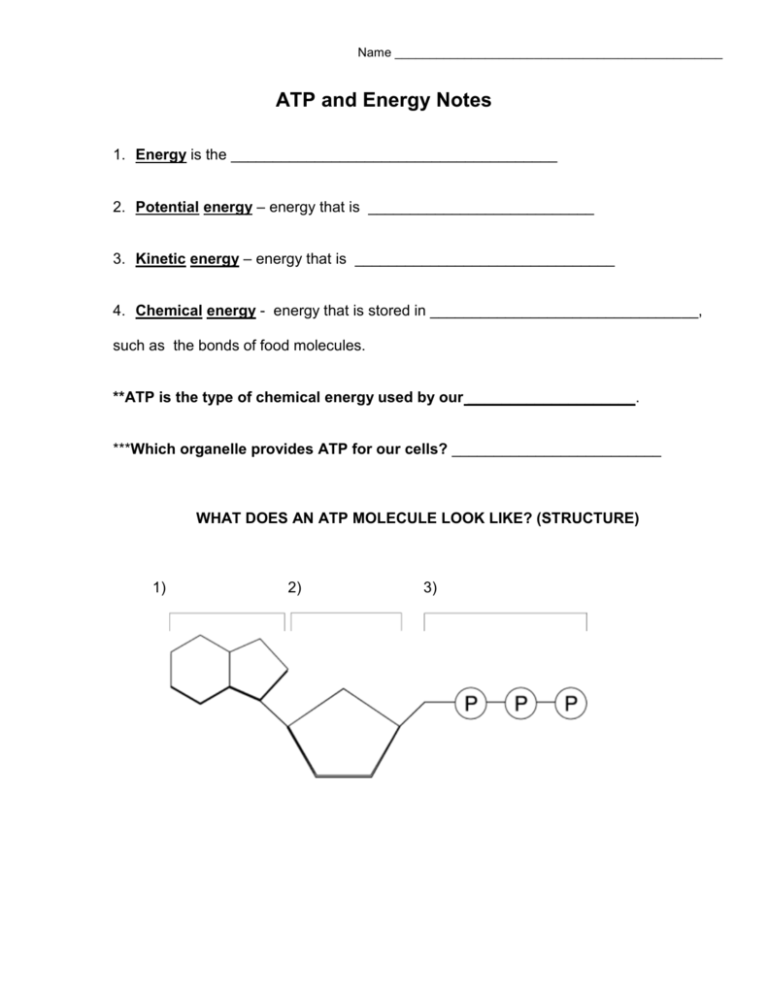
Name _______________________________________________ ATP and Energy Notes 1. Energy is the _______________________________________ 2. Potential energy – energy that is ___________________________ 3. Kinetic energy – energy that is _______________________________ 4. Chemical energy - energy that is stored in ________________________________, such as the bonds of food molecules. **ATP is the type of chemical energy used by our ____________________. ***Which organelle provides ATP for our cells? _________________________ WHAT DOES AN ATP MOLECULE LOOK LIKE? (STRUCTURE) 1) 2) 3) WHAT DOES ATP STAND FOR? ADENOSINE _________________________________ WHAT IF WE TAKE OFF ONE PHOSPHATE (p) TO MAKE ADP? ADENOSINE _______________________________ WHAT IF WE TAKE OFF TWO PHOSPHATES (P-P) TO MAKE AMP? ADENOSINE _____________________________ In other words, mono = _______, di = ________, tri = _______ WHERE DOES ATP STORE ITS ENERGY? ENERGY IS STORED IN THE ________________________________ **HOW DOES A CELL RELEASE THE ENERGY FROM THE ATP? **ONCE ENERGY HAS BEEN RELEASED, AND A PHOSPHATE IS GONE, ATP BECOMES _____________. HOW DOES THE MITOCHONDRIA MAKE MORE ATP? IT “RECYCLES” ADP BY ADDING A ______________________________________________ THIS IS CALLED THE ATP- ADP CYCLE: ATP ADP *** ALL CELLS USE ATP, BUT THEY GET IT FROM DIFFERENT SOURCES. 1. AUTOTROPHS, OR ______________________________ 2. HETEROTROPHS, OR ____________________________ All cells have ATP, but some transform light into ATP and others get ATP from food. Autotrophs - plants; _________________________ food from light energy. Heterotrophs - animals; etc.; get food and break it down to get energy (ATP) in the process of _______________________ _________________________________. A little bit of a Chemistry Review!!! Activation energy – energy needed to start a chemical reaction Enzymes – lower the amount of activation needed to start a chemical reaction Endergonic (endothermic) reactions – uses more energy than it gives off; will be cold Exergonic (exothermic) reactions – gives off more energy than it uses; will be warm