CNS 1 - 4Drs
advertisement

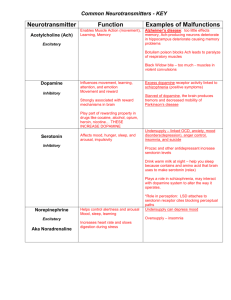
CNS 1 Biochemistry 2 Dr.Faisal Al khateeb Reem Al-kilany This sheet contains the additional notes only, there are many reactions in the slides so make sure to study the slide and the sheet hand in hand. There are few sentences written in italic and underlined, these are information from last year lecture that weren’t mentioned in ours, I’m not sure about them but they can be useful. Today we’ll look at synthesis and activation of a number of neurotransmitters, look at their role in different physiological states and try to link synthesis and activation of neurotransmitters with our understanding of biochemistry and metabolism slide 1 - our brain is so sophisticated slide2 - acetylcholine released at nerve endings leads to muscle contraction - acetylcholine has two types of receptors: muscarinic and nicotinic slide 3 - acetylcholine synthesis starts from choline and acetyl CoA - this reaction is favorable because it’s a hydrolysis reaction! - we can’t simply add acetate and choline, because acetate should be in the ACTIVE form that is acetyl CoA slide 4 - after release of acetylcholine from synaptic cleft it can be taken again “re-uptake” - low km =high affinity . -high km= low affinity Page 1 of 8 - phosphatidylcholine is a component of plasma membrane - methylation requires methyl donor “methionine” slide 5 - re uptake of acetylcholine from synapses: acetylcholine is released, it binds to Ach receptors or to acetylcholine esterase - acetylcholine esterase degrades acetylcholine to choline and acetate - uptake of choline as soon as it’s released - acetate diffuses to blood and won’t be re utilized - acetyl CoA is a product of fatty acid oxidation and glucose oxidation or catabolism - in Brain the source of acetyl CoA is pyruvate dehydrogenase reaction ( end product of glycolysis) so ONLY glucose is the source of acetyl CoA and energy in the brain , amino acids ( I think the doctor meant to say fatty acids) are not a source of acetyl CoA and this illustrates the importance of continuous supply of glucose to the brain (hydrolysis of acetyl CoA gives acetate which can’t be used again) slide 6 - agonists increase or prolong the effect of drug, antagonists block it - acetylcholinesterase inhibitors inhibit hydrolysis of acetylcholine - nicotine at low doses works as an agonist - atropine is an antagonist for acetylcholinesterase inhibitors ( which are a group of substances used in chemical weapons and pesticides) so it’s used in cases of acetylcholine intoxication or acetylcholinesterase inhibitors intoxication. Slide 7,8,9 and 10 - catecholamines are a group of compounds that contain an indole ring and are amines - all are synthesized from phenylalanine or tryrosine - synthesized by: o 1. hydroxylation of phenyl group to give tyrosine , this reaction requires oxygen atom and the other oxygen atom will be reduced by Page 2 of 8 tetrahydrobiopterin (oxidizing substance) which will be oxidized to dihydrobiopterin - tetrahydrobiopterin is a cofactor that ulternates between tetrahydrobiopterin and dihydrobiopterin - NADH is a reducing factor that prevents formation of free radicals o 2. hydroxylation reaction for tyrosine, also uses oxygen and tetrahydrobiopterin, gives DOPA ”dihydroxyphenylalanine” o 3. decarboxylation reaction for DOPA gives dopamine o 4. hydroxylation reaction that requires a different cofactor that is ascorbate (vitamin C) , this reaction gives norepinephrine from dopamine. “ here hydroxylation of an ETHYL group” o 5. methylation of norepinephrine gives epinephrine, this reaction requires a methyl group donor “SAM= S-adenosylmethionine” Notes: If the nerve utilizes dopamine, the reaction stops at the 3rd step, otherwise it’ll continue to give norepinephrine or epinephrine. Norepinephrine= No R epinephrine which means an epinephrine without R. “R indicates a methyl group” Slide 11 - condensation of methionine and ATP by methionine adenosyl transferase gives SAM - SAM is a methyl group donor, and methylation reaction is important in synthesis and degradation of neurotransmitters, after methylation reaction SAM becomes Sadenosyl homocystiene , and in order to regenerate SAM vitamin B12 and folic acid are required, that’s why a deficiency in vitamin B12 and\or folic acid lead to neurological disorders Slide 12 - 1st step in the synthesis of dopamine by tyrosine hydroxylase occurs in the cytosol - Dopamine transport to a storage vesicle requires ATP “active transport” Page 3 of 8 - Inside storage vesicle dopamine will be converted to norepinephrine by DBH (Dopamine Beta Hydroxylase) Slide 13 - Rate of firing and rate of synthesis should be balanced, thus the reactions - of catecholamine synthesis- occur in several steps and mechanisms. - Because tyrosine hydroxylase is inhibited by free cytosolic catecholamines (high level of catecholamines in the cytosol) norepinephrine and epinephrine synthesis takes place in the storage vesicles - BH4= tetrahydrobiopterin - tyrosine hydroxylase activity increases by depolarization of nerve terminals - protein kinases phsophorylate TH (tyrosine hydroxylase) itself. - This is the short term regulation Slide 15 - long term regulation - prolonged sympathetic activity by continuous firing of catecholamines - tyrosine hydroxylase AND dopamine hydroxylase are two major enzymes for dopamine and norepinephrine synthesis - CREB is a transcription factor - Phosphorylated CREB binds to CRE promoter region of the gene CREB (cAMP response element-binding) gene transcription takes place increased level of mRNA. Slide 16 - two major enzymes in degradation : monoaminoxidase and catechol-omethyltransferase. Slide 17 - monoaminoxidase contains 1 amine group Page 4 of 8 - MAO works by: 1. deamination of ammonia 2. oxidation to aldehyde group - MAO-A is present in the CNS - MAO-B is present in the liver and small intestine, protects against biogenic amines, which are substances found in some food especially cheese and wine, degradation of theses substances (biogenic amines) produces tyramine which has a toxic effect. Slide 18 - COMT inactivates catecholamines by adding a methyl group (methylation) - MAO and COMT are widely distributed, any of these two enzymes can start the deactivation process, it’s not necessary to work in a certain order. This emphasizes the importance of the inactivation process. Slide 19 - shows a large number of intermediates :S slide 20 - VMA is measured in CSF, CNS, urine to measure the amount of epinephrine in the body. - VMA is present in vanilla, so avoid eating vanilla or banana before measuring it . “banana contains vanilla !” Slide 22 - synthesis of serotonin starts from tryptophan, enzyme: hydroxylase “this enzyme utilizes oxygen and tetrahydrobiopterin as a cofactor”. - 1st step is a hydroxylation reaction, adding a hydroxyl group to the indole ring (similar to 3rd step in dopamine synthesis.) Slide 23 - degradation of serotonin also by MAO followed by oxidation Page 5 of 8 - amine group is converted to aldehyde group ( by MAO) - aldehyde group is converted to carboxylic acid (by oxidation) - 5HT (5-hydroxy tryptamine) is serotonin Slide 24, 25 and 26 - depression is associated with serotonin deficiency. - we give MAO inhibitors to elevate the level of serotonin and treat depression - drugs that treat depression either prevent reuptake ( increase level of monoamines in the synapse) or inhibit degradation (MAO inhibitors) - selective serotonin re-uptake inhibitors have less side effects - undersupply of dopamine leads to Parkinson disease - high level of dopamine gives a pleasant satisfied feeling. - Cocaine blocks reuptake of dopamine and gives a pleasant satisfied feeling. - Increased concentration of amphetamine or cocaine decrease the amount of receptors as a regulatory mechanism and this decreases the sensitivity, this decrease in sensitivity leads to drug addiction because you start increasing the dose in order to compensate for the low sensitivity. - DOPA alone is not sufficient as a treatment for Parkinson because it’s rapidly metabolized, we give MAO inhibitors in addition to DOPA. Slide 27 and 28 - the figure on your right hand is histidine - synthesis of histamine from histidine starts by decarboxylation reaction which requires PLP (vitamin B6) - inactivation of histamine by methylation - regeneration of SAM requires vitamin B12 and folic acid - notice that the pathway of neurotransmitter synthesis is a short one like any regulatory signal that should be easily produced and easily deactivated. - Histamine is present all over the body because it’s not only a neurotransmitter but it’s also released in cases of allergy Page 6 of 8 Slide 29 and 30 - glutamate synthesis by amination or transamination - starting from glucose to pyruvate to citric acid cycle to alpha-ketoglutarate, which –by amination- will be converted to glutamate - glutamine by transamination (glutaminase) will be converted to glutamate - elimination of glutamate by high affinity uptake to nerve terminal and glial cells ( glial cells provide nutrition for the neuron) - Non NMDA receptors do not bind aspartate Slide 31,32 and 33 - GABA (gamma amino butyric acid) is produced from glutamate by a decarboxylation reaction - To regenerate glutamate from GABA u can’t simply carboxylate GABA, it requires an indirect process known as GABA shunt - The GABA shunt: 1. removal of amino group require continuous supply of alphaketo glutarate, this converts an amine to an aldehyde (semi aldehyde) then to succinate.* check the last page - Vitamin B6 is required for decarboxylation and transamination reactions. Slide 34 - glycine is a small amino acid - serine can be converted into glycine by a decarboxylation reaction slide 35 - nitric oxide is the only neurotransmitter in the form of gas. - it’s not only a neurotransmitter, it works in the peripheral tissue as a smooth muscle relaxant, prevents platelet aggregation and has a bactericidal activity - synthesis of nitric oxide starts from the amino acid arginine then removal of amine group by the enzyme NO synthase gives citrulline and NO is released. “note that this reaction also requires O2 and NADPH” Page 7 of 8 - mechanism of action of NO: it enters the cell, stimulates guanylate cyclase which converts GTP into c-GMP, and c-GMP activates protein kinases that decrease the interaction between actin and myosin leading to smooth muscle relaxation. This is the mechanism of action of nitroglycerine – a drug used for treating angina- which releases NO and thus relaxes smooth muscles. - Phosphodiesterase is an enzyme which converts c-GMP to GMP, therefore phosphodiesterase inhibitors prevent c-GMP degradation. - Phosphodiesterase inhibitors “eg: viagra” are contraindicated in patients using nitroglycerin because of their severe adverse reactions. - Being a gas, NO doesn’t depend on the classical way that is release from presynaptic terminals and binding to receptors on post synaptic terminals, it can diffuse between cells, from presynaptic terminal to post synaptic terminal or from post synaptic to presynaptic or from cell to cell , it doesn’t require receptors. * here the recording wasn’t clear so I had to include an additional information about GABA: - Since GABA does not penetrate the blood brain barrier, GABA is, therefore, synthesized in situ. It is synthesized from glutamate using the enzyme L-glutamic acid decarboxylase and pyridoxal phosphate (which is the active form of vitamin B6) as a cofactor via a metabolic pathway called the GABA shunt. This process converts glutamate, the principal excitatory neurotransmitter, into the principal inhibitory neurotransmitter (GABA). - Pyridoxal phosphate, a coenzyme, the active form of vitamin B6 Sorry for being late, but the slides were available in Al Ra2ed on Wednesday and I had them on Thursday. Special thanks for Eshraq Al Qadri for giving me last year’s slides GOD gave you many reasons to be happy, try to concentrate on them. Thank you, Good luck. The end I wonder why he dislikes physiology and physiologists !! 8-O Page 8 of 8