RCT Worksheet - teachepi.org
advertisement

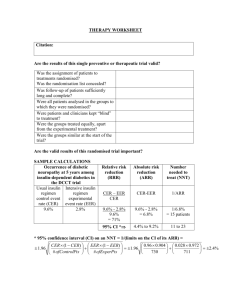
CRITICAL APPRAISAL OF A TRIAL RCT WORKSHEET Citation: Are the results valid? 1. Was there a clearly defined, focused research question? What was the study question? 2. Was the assignment of patients to treatments randomised? -Was randomisation (allocation) concealed? 3. Were all patients who entered the trial accounted for at its conclusion? -and were they analysed in the groups to which they were randomised (intention-to-treat analysis)? 4. Were subjects in the treatment and control groups similar with respect to known prognostic variables? 5. Were patients aware of group allocation? 6. Were clinicians aware of group allocation? 7. Were outcome assessors aware of group allocation? Source: Adapted from 1) Centre for Evidence Based Medicine (http://www.cebm.net/); 2) Badenoch & Heneghan, Evidence-based Medicine Toolkit, BMJ Books, 2002; and 3) Guyatt & Rennie. Users’ Guides to the Medical Literature, AMA Press, 2002. Compiled by Madhu Pai [madhukar.pai@mcgill.ca] 1 CRITICAL APPRAISAL OF A TRIAL RCT WORKSHEET 8. Was duration of follow-up adequate? Was follow-up complete? Any other potential biases in this study? Potential for selection bias? Potential for information bias? Potential for confounding? Was there a clear rationale for the sample size/power estimation? What were the primary and secondary endpoints of the study? How well were the endpoints measured? Were surrogate endpoints used? Were the data analytic methods appropriate for the research question and study design? Source: Adapted from 1) Centre for Evidence Based Medicine (http://www.cebm.net/); 2) Badenoch & Heneghan, Evidence-based Medicine Toolkit, BMJ Books, 2002; and 3) Guyatt & Rennie. Users’ Guides to the Medical Literature, AMA Press, 2002. Compiled by Madhu Pai [madhukar.pai@mcgill.ca] 2 CRITICAL APPRAISAL OF A TRIAL RCT WORKSHEET What are the results? SAMPLE CALCULATIONS: Occurrence of endpoint (outcome) Control Event Rate [CER] Experimental Event Rate [EER] 9.6% 2.8% Relative Risk Reduction RRR CER - EER CER 9.6% - 2.8% 9.6% = 71% Absolute Risk Reduction ARR CER - EER Number Needed to Treat NNT 1/ARR 9.6% - 2.8% = 6.8% (4.3% to 9.3%) 1/6.8% = 15 pts, (11 to 23) 95% Confidence Interval (CI) on an NNT = 1 / (limits on the CI of its ARR) = YOUR CALCULATIONS: CER EER Relative Risk Reduction RRR CER - EER CER Absolute Risk Reduction ARR CER - EER Number Needed to Treat NNT 1/ARR What are the trial results? 1. How large was the treatment effect (e.g. relative risk reduction and absolute risk reduction)? 2. How precise was the estimate of the treatment effect (i.e. confidence intervals around the point estimates or p-values)? Source: Adapted from 1) Centre for Evidence Based Medicine (http://www.cebm.net/); 2) Badenoch & Heneghan, Evidence-based Medicine Toolkit, BMJ Books, 2002; and 3) Guyatt & Rennie. Users’ Guides to the Medical Literature, AMA Press, 2002. Compiled by Madhu Pai [madhukar.pai@mcgill.ca] 3 CRITICAL APPRAISAL OF A TRIAL RCT WORKSHEET Can you apply the results to patient care? 1.Were the study patients similar to the patient in your practice? -Does your patient match the study inclusion criteria? -If not, are there compelling reasons why the results should not apply to your patient? 2. Were all clinically important outcomes considered? 3. Are the benefits worth the costs and potential risks? -What is the number needed to treat (NNT) to prevent one adverse outcome or produce one positive outcome? -Is the reduction of clinical endpoint worth the increase of cost and/or risk of harm? In summary: What are the major strengths of this study? What are the major limitations of this study? Are there any major ethical concerns with this study? Source: Adapted from 1) Centre for Evidence Based Medicine (http://www.cebm.net/); 2) Badenoch & Heneghan, Evidence-based Medicine Toolkit, BMJ Books, 2002; and 3) Guyatt & Rennie. Users’ Guides to the Medical Literature, AMA Press, 2002. Compiled by Madhu Pai [madhukar.pai@mcgill.ca] 4