1. policy - HealthcareGovernance.org.au
advertisement

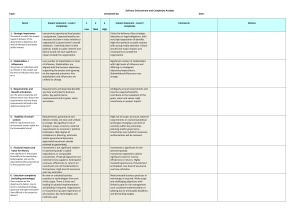
Appendix 6 LOGO Model Community Health Service CLINICAL GOVERNANCE POLICY AND PROCEDURES Approval Date: Review Date: Page x of xx Authorised by: DRAFT 1. POLICY Model Community Health Service is committed to ensuring that clients receive high quality services. The Board of Management is responsible for ensuring good clinical governance, and the CEO, Managers, Staff, Department of Human Services, and Community all have a role to play in the process. 2. PURPOSE AND SCOPE The purpose of the clinical governance policy is to provide a framework to ensure that all practitioners employed at Model Community Health Service provide a high quality service. It will ensure that systems are in place to support, evaluate, and report on safety and quality across the organisation. The policy defines levels of responsibility across the whole organisation. 3. REFERENCES Better Quality, Better Health Care - A Safety and Quality Improvement Framework for Victorian Health Services. The Victorian Quality Council DHS 2003. Enabling the Consumer role in clinical governance. The Victorian Quality Council DHS, 2004. The Healthcare Board’s role in clinical governance- The Victorian Quality Council DHS, 2004 Leading clinical governance in health services: The Chief Executive Officer and Senior Manager roles. The Victorian Quality Council DHS, 2005 Developing the clinical leadership role in clinical governance: A guide for clinicians and health services. The Victorian Quality Council DHS, 2005 Health and Community Services Core Module. Quality Improvement Council (QIC) 2004 Occupational Health and Safety Act 2004 Model CHS Board of Management Clinical Governance Policy Other relevant CHS Policies eg. Client Access Policy Assessment and Care Policy Client Record Policy Recruitment Policy Performance Appraisal Policy Staff Development Policy Occupational Health and Safety Policy Service Review Policy Client and Community Engagement Policy etc… 4. DEFINITIONS Clinical Governance A “framework through which health organisations are accountable for continuously improving the quality of their services and safeguarding high standards of care by creating an environment in which excellence in clinical care will flourish” Sir Liam Donaldson, NHS Chief Medical Officer “The responsibility of governing bodies to demonstrate sound strategic and policy leadership in clinical safety and quality, to ensure appropriate safety and quality systems are in place, and to ensure organisational accountability for safety and quality.” Dr Heather Wellington Appendix 6 Features of a quality organisation A quality organisation is Efficient, Legal, Accountable, Sustainable, Participatory, Reflexive, Integrated, and its services and programs are Effective, Competent, Safe, Accessible, Fair, Responsive, Inclusive and culturally sensitive, Coordinated and has a culture of continuous quality improvement. Quality Improvement Council 2004 5. CLINICAL GOVERNANCE FRAMEWORK Model Community Health Service is a complex organisation with staff from a range of health and community service professional backgrounds. To ensure a system of safety and quality across the service, a clinical governance framework has been developed that each discipline will use to develop, implement, monitor, improve and report quality and safety activities. This framework is based on the Victorian Quality Council and QIC guidelines. It outlines four key organisational elements and six quality dimensions to be considered. The framework takes a systems approach, ensures a systematic and comprehensive reporting process, and will ensure that action will be taken on issues identified in a timely manner. Some aspects of the framework involve organisation wide structures and processes whilst others are more relevant at the discipline level. ORGANISATIONAL ELEMENTS Governance, Leadership & Culture This includes the structures and processes put in place by the board to meet its governance obligations and can include: Governance Policies Organisational Structure Strategic & Operational Planning Planning & Evaluation Cycle Board Reporting requirements Delegation of Authority – Financial, Staffing, General Consumer and Community Involvement This includes consumers of the service, their families, carers, members and the community generally. Processes to achieve include: Elected Board Members Client satisfaction surveys and focus groups Client involvement in service reviews Client complaints processes Competence of and education to support service providers This includes competencies of the organisation as a whole, as well as specific teams, and individuals who deliver services. Processes in place to achieve this include: Recruitment of qualified, experienced professionals Annual performance appraisals for all staff Professional development Staff supervision Credentialing Information Management & Reporting This includes collection of data, technology necessary for data collection, the reliability and validity of the data and how it is reported and used. To support the processes, data should be available, accurate, timely and relevant. Processes include: Complaints reporting Incident reporting Activity reporting Appendix 6 Wait List reporting Strategic & Operational Plan reporting Client Records Audits DIMENSIONS OF QUALITY Safety Clients should be safe both in the environment and with the treatment they receive. All potential safety risks need to be identified and processes developed to eliminate or minimise the risks. This is achieved through: Accreditation audits (QICSA / HACC / GP) Audits – eg. Infection control / Food Safety / Drug Storage Incident review Complaints review Effectiveness Clients expect to benefit from any treatment they receive. To evaluate this, outcome measures need to be used. A range of measures appropriate for the service provided should be considered. Measures include: Clinical Indicators Client satisfaction surveys Client complaint processes Appropriateness Practitioners need to provide services based on evidence so that the right intervention occurs for the clients at the appropriate time. Processes to ensure this include use of: Clinical pathways Standardised assessment tools Client care plans Client record audits Acceptability Acceptability describes whether or not the service meets the needs and expectations of the range of informed consumers. Processes in place for achieving this dimension include: Strategic Planning (includes community and consumer consultation) Complaints monitoring Client satisfaction surveys and focus groups Staff professional development Access Access to services should be equitable across the catchment area and for different community groups. Processes in place to support access include: Standardised intake systems Service information Interpreter systems Outreach services Demand management systems Fee Policy Accessible buildings Efficiency Efficient use of resources includes examining both cost of services and benefits to consumers. Current processes addressing efficiency at DCH include: Service planning, reporting and evaluation frameworks Financial monitoring Appendix 6 For all of the quality dimensions listed above, each discipline will identify relevant issues, and develop systems to support monitoring, identifying continuous improvement opportunities, and mechanisms for reporting on the area. 6. LEVELS OF RESPONSIBILITY Board of Management have overall responsibility to ensure that the organisation has a quality and safety management system in place, and to receive regular reports against this system CEO & Management Team have responsibility for development, implementation and review of the system Practitioners have responsibility to be involved in the development, implementation, review and reporting of the system related to their work Consumers, the community and other stakeholders should be involved in the process as appropriate Different CHSs will choose the appropriate methodology through which the responsibilities for clinical governance are enacted. This may be achieved through a Quality Committee or the existing management meeting structures. 7. PLANNING AND REVIEW Model Community Health Service undertakes a comprehensive planning and review process based on a three yearly cycle. This is monitored annually via a Planning, Reporting & Evaluation Schedule. The Strategic Plan is reviewed annually leading to the development of annual Operational Plans which are monitored quarterly. 8 INDIVIDUAL SERVICES Each service undergoes a full review every three years. This review will focus on all the dimensions of quality. A summary of this review plus any resulting recommendations are forwarded via the management group to the Board of Management. In addition to the above review, each service will develop a report for the Board of Management annually that outlines current safety & quality processes and intended quality & safety improvement initiatives. 9 EXTERNAL QUALITY ASSURANCE PROGRAMS Model Community Health Service undergoes a number of external formal accreditation programs. These occur in three year cycles commonly with mid-cycle review processes. They include Quality Improvement Council Service Accreditation (QICSA), Home and Community Care (HACC) National Standards Accreditation, General Practice (GP) accreditation, other systems. 10 BOARD REPORTING A variety of reports are forwarded to the Board for consideration. These include: Strategic plan review Annual operational plan Operational plan progress reports Service quality & safety system processes Service review reports Audit of client complaints Quality improvement reports Adverse event reports Activity reports Annually Annually Quarterly All services once per year All services once every three years Six monthly As developed As occur Quarterly Appendix 6 Wait list reports 11 Quarterly DOCUMENTS Board Reporting Proforma. 12 Date HISTORY Initial Issue Appendix 6 Name and logo of community health service Approval date: Clinical Risk Management Policy and Procedure Authorised by: 1. Review Date Page x of xx DRAFT Jan-06 POLICY The Community Health Service is committed to providing clients with high quality clinical services. This is achieved by ensuring that clinical governance systems are in place including a Clinical Risk Management Framework. The responsibility for clinical governance lies with the Board of Management, through the CEO, with input from management, staff, the Department of Human Services (DHS) and the community. 2. PURPOSE AND SCOPE The purpose of this policy is to provide a structured approach to clinical risk management within a clinical governance framework that ensures that all practitioners employed at the Community Health Service provide a high quality clinical service. Systems have been developed that will ensure that potential clinical risks are identified, analysed, evaluated and subsequently eliminated, reduced or controlled. Both managers and clinical staff are involved in this annual process. 3. 4. REFERENCES Clinical Risk Management Guidelines for the Western Australian Health System Information Series No. 8, Department of Health, Government of WA 2005 Australia/New Zealand Standard on Risk Management AS/NZS 4360:2004 Community Health Service Policies o Risk Management Policy o Clinical Governance Policy o OHS Policy o Other relevant policies DEFINITIONS RISK The exposure to the possibility of such things as economic or financial loss or gain, physical damage, injury or delay, as a consequence of pursuing a particular course of action. The concept of risk has two elements: the likelihood of something happening and the consequences of it. RISK ANALYSIS The systematic use of available information to determine how often specified events may occur and their likely consequences. The purpose of risk analysis is to identify the causes, effects and magnitude of risk and provide a basis for risk assessment and risk treatment. RISK ASSESSMENT The processes used to determine risk management priorities by evaluating and comparing the level of risk against organisational standards, predetermined target risk levels or other criteria. RISK The process of determining what can happen, why and how. Appendix 6 IDENTIFICATION RISK MANAGEMENT The systematic application of management policies, procedures and practices to the task of identifying, analysing, assessing, treating, monitoring and communicating risk. RISK TREATMENT The selection and implementation of appropriate management options for dealing with identified risk. 5. PROCEDURE To ensure a system of safety and quality across the service, a clinical risk management framework has been developed that each discipline will use to develop, implement, monitor, improve and report quality and safety activities. The five-step Clinical Risk Management process is based on the risk management process outlined in the Australian and New Zealand Standard (AS/NZS) 4360:2004. These are: 1. 2. 3. 4. 5. Establishing the Context Clinical Risk Identification Clinical Risk Analysis Clinical Risk Evaluation Clinical Risk Treatment The framework takes a systems approach, ensures a systematic and comprehensive reporting process, and will ensure that action will be taken on issues identified in a timely manner. Each discipline will be responsible for developing its own clinical risk framework, which will then feed into an organisational risk register. In addition, monitoring and review are essential components of the risk management process. It is essential that clinical risks and risk treatment plans, strategies and management systems are continually monitored to ensure that the organisation is able to control the implementation of risk treatments. 5.1 Establishing the context The goals, objectives, strategies, scope and parameters of the activity, or part of the organisation to which the clinical risk management (CRM) process is being applied, should be established. To assist in this process, each discipline or program area should engage in an annual planning process or service plan. This will identify clinical processes that take place within the service and assist in identification of actual or potential risks. 5.2 Clinical risk identification Risk identification seeks to identify the clinical risks that need to be managed. A comprehensive identification system using a well-structured systematic process is critical, because a potential risk not identified at this stage will be excluded from further analysis and treatment. There are a number of methods for identifying clinical risks. These include: sentinel event reporting; incidents reporting; complaints data; flow charting/systems design review; audits or physical inspections; SWOT analysis; networking with peers, professional groups and industry bodies; FOI requests or medico-legal data. Appendix 6 Key questions to be asked include: o What, when, where, why and how are the risks likely to occur and who might be involved? o What is the source of each risk? o What are the consequences of the risk? o What are the health service’s external and internal obligations? o Is any further research needed into specific risks? o How reliable is the information? o Have the right people been involved in the risk identification process? o Can it be benchmarked with peer organisations? 5.3 Clinical risk analysis Analysis of identified risks is a systematic process that seeks to understand the nature of the clinical risk. During this process, the likelihood and consequence of each clinical risk are then examined in detail and a risk rating is given according to a Risk Assessment Matrix. Risks may be rated as low, moderate, high and extreme and prioritised accordingly. Steps in this process are: 1. 2. 3. 4. Determine the adequacy of existing controls Determine the likelihood of the clinical risk Determine the potential consequences of the clinical risk Calculate the level of clinical risk according the following formula Risk level = Consequence x likelihood 5.4 Clinical Risk Evaluation Clinical risk evaluation involves comparing the level of risk found during the analysis process with previously established risk criteria. The output of a clinical risk evaluation is a prioritised list of risks for further action. Once the risks are ranked and measured, it should be determined which of the risks the organisation is prepared to accept and at which level of the organisation they should be managed. Other options for risk treatment include referral of the risk to a higher authority for acceptance, amending the activity or task so that the level of risk is reduced, or lastly, to cancel the activity or task. 5.5 Clinical Risk Treatment Clinical risk treatment involves identifying the range of options for treating clinical risk, assessing those options, preparing risk treatment plans and implementing them. Where risks cannot be eliminated, a combination of treatment options should be applied to control or treat the risks to the maximum extent possible. Each treatment option should be evaluated for effectiveness. Alternative treatment options may include 1. Avoiding the clinical risk o not proceeding with the activity where the risk is occurring 2. Reducing the level of clinical risk Appendix 6 o reducing either the consequences or the likelihood of the risk through enhancement of existing controls or additional controls o it is essential that the organisation provide the resources for risk reduction as required 3. Transferring the clinical risk o the risk may be shared with another party (transferred by contract, administrative processes etc) 4. Retaining the clinical risk o in instances where it is too costly or impossible to avoid, reduce, transfer or eliminate the risk, the decision to retain the risk should be documented and listed on a centralised risk register o periodic monitoring is essential o contingency plans may be required Documentation It is essential for an appropriate level and standard of documentation to be maintained. This will ensure correct clinical risk management process; enable decisions, action plans and processes to be communicated internally and externally as required; demonstrate an appropriate audit trail and provide all staff with accurate information regarding the organisation’s risk management processes. Monitoring and review Monitoring and review of the organisation’s clinical risk management framework is an essential part of the process. New clinical risks need to be identified and old clinical risks should be treated and then removed form the register as required. Monitoring may include internal or external audits, performance reviews, reviews of incident and investigation reports, review of organisational strategies and policies and program evaluation. It is recommended that a formal internal review take place annually. 6. DOCUMENTS o 7. DATE Clinical Risk Management Framework for Community Health Services in Victoria HISTORY Initial Issue