1 - SCH4U1-05-2010
advertisement

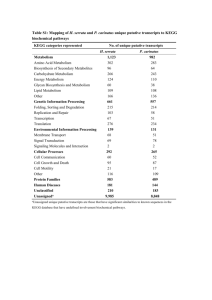
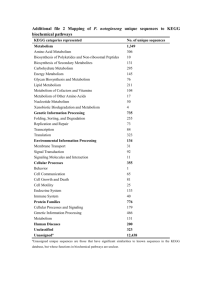
Hassti & Amelia Applications of Chemistry in…human metabolism! Hassti’s Connections - #1 – 3 (energy and change, rate of chemical reactions, redox reactions) Amelia’s connections - #4 – 6 (Acids/bases, Le Chatelier’s Principle, Entropy) Metabolism refers to the set of chemical processes that happen in living organisms to maintain life. It is the sum of all anabolic and catabolic processes in an organism. These processes allow organisms to grow, maintain their structures, and reproduce. Metabolism is divided into two components: catabolism and anabolism. Catabolic reactions are processes that break macromolecules into constituent individual subunits, while anabolic reactions are processes that produce large molecules from smaller subunits. It seems as though human metabolism is solemnly concerned with biology. However upon greater research, it is shown that this topic is in fact much related to chemistry. Here is a summary of six ways as to how human metabolism relates to chemistry. 1. Energy and Change -human metabolism is a series of chemical reactions that involve energy change and transfer -there are two main types of chemical reactions that are involved in human metabolism: endothermic and exothermic reactions -an exothermic reaction is a chemical reaction in which the energy of the products is less than the energy of the reactants, in other words there is a net release of energy -an endothermic reaction is a chemical reaction in which the energy of the products is more than the energy of the reactants, in other words there is a net absorption of energy -two main chemical reactions in metabolism that illustrate this are as follows: Note: Only cellular respiration takes place in human metabolism. Photosynthesis occurs only in plants. Cellular respiration: exothermic reaction -- ∆H < 0 C6H12O6 + 6O2 6CO2 + 6H2O ∆H = -2870 KJ/mol C6H12O6 used Photosynthesis: endothermic reaction -- ∆H > 0 6CO2 + 6H2O C6H12O6 + 6O2 ∆H = +2870 KJ/mol C6H12O6 formed According to the 1st law of thermodynamics, energy cannot be created nor destroyed; it can be changed from one form to another or transferred from one object to another. Cellular respiration is the process that traps and stores the free energy from the foods we eat. How is energy converted from one form to another in the body you may ask? Well the answer is ATP! ATP is like a rechargeable battery. Energy is released when ATP (charged form) is converted into ADP (uncharged form). The energy is then used to power endothermic reactions in the body. In other words, the energy is released to do work. 2. Rate of Chemical Reactions -the chemical reactions involved in metabolism occur under specific rates -the human body has several mechanisms to increase the rate of the body’s chemical reactions ---one of which is the usage of biological catalysts, aka. Enzymes—since the addition of heat would prove inefficient because the temperature of the body must stay within a limit -an enzyme is a biological catalyst (a substance that is used to increase the rate of a chemical reaction without being consumed in the reaction) -an enzyme increases the rate of chemical reactions involved in metabolism by lowering the activation energies that are needed to trigger the reaction; therefore the number of reactants that have sufficient energy to react are increased -an example of such an enzyme involved in human metabolism is lactase which is responsible for breaking down lactose, a sugar found in milk -lactase is found in the small intestines, liver and the kidneys and it catalyzes the breakdown of lactose to simple sugars, glucose and galactose Hassti & Amelia -lactase lowers the activation energy by providing an alternative mechanism for the reaction because according to Hess’s Law the enthalpy change of a physical or a chemical process depends only on the beginning conditions (reactants) and the end conditions (products); it does not depend on the pathway or the reaction mechanism 3. Redox Reactions -reaction that involves the transfer of electrons -oxidation is the reaction that involves the loss of electrons -reduction is the reaction which involves the gaining of electrons -a reaction that involves both oxidation and reduction is called an oxidation-reduction reaction, also known as a redox reaction -there are various redox reactions that occur in human metabolism -the following are some examples: -the electron transport chain -reactions involving the reduction or oxidation of coenzymes such as NAD+ and FAD -for instance, the reduction of NAD+ to NADH involves the transfer of a hydride ion which contains two electrons NAD+ + 2 e- + 2 H+ <--> NADH + H+ -oxidative phosphorylation Cellular respiration: exothermic reaction -- ∆H < 0 C6H12O6 + 6O2 6CO2 + 6H2O ∆H = -2870 KJ/mol C6H12O6 used -this is an oxidation process because hydrogen atoms carry electrons away from carbon atoms in glucose to oxygen atoms -when the hydrogen atoms form covalent bonds with oxygen, the shared electron pairs occupy positions closer to the oxygen nuclei than they did when they were part of the glucose molecule since oxygen is much more electronegative than carbon -example of a main redox reaction/process involved in metabolism: electron transport chain (ETC) --composed of a series of membrane-associated protein complexes arranged in order of increasing electronegativity --electrons are transferred to the first protein from coenzymes (NADH and FADH 2) --electrons are then transferred between the proteins --each component is reduced by gaining two electrons from the component before it in the chain and oxidized by losing two electrons to the component after it in the chain --the ultimate goal of this redox reaction is to create an electrochemical gradient (by pumping hydrogen ions across the membrane) that is used to make more ATP (thereby harvesting energy) Hassti & Amelia 4. Acids/bases, buffer systems in human metabolism As we ingest foods, the process of digestion sets off a series of reactions in our body. Stomach acidity increases, but that same acidity must be neutralized when the digested foods pass on to the small intestines. That becomes the jobs of our bodies’ amazing buffer systems. A buffer is any solution that contains a weak acid with its strong conjugate base, or a weak base with its strong conjugate acid. Stomach acid is neutralized by the sodium bicarbonate buffer system HCl H+ + ClNaHCO3 Na+ + HCO3HCO3- OH- + CO2 Na + OH + CO2 + H + Cl H2O + NaCl + CO2 HCl + NaCO3 H2O + CO2 + NaCl 5. Le Chatelier’s principle Recall this reaction from earlier CO2(aq) + H2O(l) H2CO3(aq) H+(aq) + HCO3-(aq) As our body tissue release H+ into our blood, the reaction shifts towards the left, increasing [H2CO3] and [CO2], the pressure from CO2 builds and we exhale, getting rid of the excess CO2 and restoring balance. As our body tissue remove H+ from our blood, the reaction shifts towards the right, decreasing [H2CO3] and [CO2], the pressure from CO2 drops and we inhale, restoring CO2. 6. Entropy -As we touched upon earlier in the thermodynamics unit, all reactions increase entropy. Here we will take a look at … -how even though as we age, we put random things into organized parts such as food into muscle; we are still increasing the chaos level of the universe. -How the change of breaking down food is larger than the order we create by using those broken down bits to repair cells, build muscle etc. -How through metabolism, we are ironically contributing towards our deaths via increased entropy of our body, but still needing to do so. Hassti & Amelia References -Hassti Cellular metabolism . (n.d.). Retrieved from http://www.biologyinmotion.com/atp/index.html Christian, E. (n.d.). Chemistry of Metabolism . Encyclopedia of diet . Retrieved from http://chestofbooks.com/health/nutrition/Diet Encyclopedia/Lesson -V I-Chemistry-Of-Metabolism.html Cunningham, E. (1978). Biochemistry: mechanisms of metabolism . South Carolina: McGraw -Hill Book Company. Energy transfer in metabolism. (2002). Retrieved from http://staff.jccc.edu/PDECELL/metabolism/ent rans.html Gropper, S., Groff, J., & Smith, J. (2009). The Digestive System: Mechanisms for Nourishing the Body. Advanced nutrition and human metabolism 5th ed (pp. 24-28). Wadsworth, US: Cengage Learning. The role of enzymes in metabolism . (n.d.). Retri eved from http://www.titaneducation.com.au/samples/Biohsctr.pdf Hassti & Amelia References -Amelia Ben-Naim, A. (2007). Entropy demystified : the second law reduced to plain common sense. Hackensack: World Scientific. Chemical of the Week -- Biological Buffers. (n.d.).Science is Fun in the Lab of Shakhashiri. Retrieved April 29, 2011, from http://scifun.chem.wisc.edu/chemweek/biobuff/biobuffers.html Gropper, S., Groff, J., & Smith, J. (2009). The Digestive System: Mechanisms for Nourishing the Body. Advanced nutrition and human metabolism 5th ed (pp. 24-28). Wadsworth, US: Cengage Learning. pH Buffers in the Blood. (2008, September 5). Washington University Department of Chemistry. Retrieved April 23, 2011, from http://www.chemistry.wustl.edu/