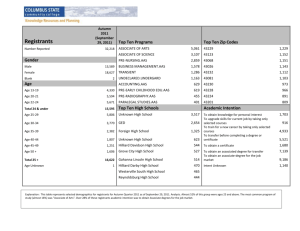
TEST506 Teacher Guid..
advertisement

Laboratory Operations Toolbox Teacher Guide PMLTEST506 Apply Spectrometric Techniques Description This unit of competency covers the ability to apply spectrometric techniques to analysis of materials. The sections concentrate on the principles of operation and testing related to an ultraviolet-visible (UV-Vis) spectrophotometer and an atomic absorption (AAS) spectrophotometer. The learner also gains exposure to other spectrometric instruments, specifically the mass spectrometer, infra-red (IR) spectrophotometer and the nuclear magnetic resonance (NMR) spectrometer. The unit is presented in 6 sections: Prepare for Analytical Procedures Spectroscopic Concepts UV-Vis Spectroscopy - Principles and Analysis Atomic Absorption Spectrometry – Principles and Analysis Structural Elucidation Using IR, NMR and MS Techniques Final Assessment Unit relationship to competency Element of competency Section in Toolbox 1. Prepare samples Prepare for Analytical Procedures Underpinning knowledge 2. Perform analytical procedures Spectroscopic Concepts UV-Vis Spectroscopy - Principles and Analysis 3. Report and communicate test results 2. Perform analytical procedures 3. Report and communicate test results 2. Perform analytical procedures 3. Report and communicate test results Atomic Absorption Spectrometry – Principles and Analysis Structural Elucidation Using IR, NMR and MS Techniques Final Assessment Teacher Guide PMLTEST506 Apply Spectrometric Techniques 1 Laboratory Operations Toolbox Teacher Guide Section: 1. Task: 1. Step: 1 Activity: Assignment - Identification of Samples and Standard Methods Questions Based on the information in MTH01SOP: Issue and Use of SL Numbers, answer the following questions. 1. Describe in your own words how SimuLab manages sample labels and SL numbers. 2. How would you maintain identification if it was necessary to divide up the contents of a sample container once testing was underway? 3. Correct labelling is critical. Describe how labelling mistakes could occur in the laboratory? Image some possible outcomes (with examples) for the customer and the laboratory if mistakes happen? Answers 1. The student should be able to provide a general description of the steps involved with the labelling process at SimuLab. This should include the correct names of relevant forms (eg. Request for Testing slip), the precaution that SL numbers should only be used once and how sub-samples are managed. A sample answer could include the information “By putting a unique identifying number on each sample and the same number on associated paperwork”. 2. Ensure that any sub-samples taken from the original sample are clearly labelled with sufficient information that would unambiguously identify the sub-sample or enable it to be linked to the original. The actual labelling could be a manually or a computer-produced ‘stick on’ label or permanent ‘texta’ markings on the container itself. 3. Look for imagination and clear thinking in this answer. Possible labelling mistakes could include such things as: Placing labels on the wrong samples Placing labels on the lids of sample containers and subsequently mixing up the lids once the testing work commences Reusing a sample container without removing the previous label. Lack of concentration and checking, not following SOP’s. Outcomes of labelling mistakes for a laboratory: labelling mistakes can result in incorrect results being reported to the customer leading to possible loss of business, tarnished reputation, legal action, wasted time including re-testing and investigating how the problem occurred and wasted resources including chemicals and equipment. Outcomes of labelling mistakes for a customer: labelling mistakes mean incorrect results which may lead to undesirable impacts in many areas such as OHS, product safety, lost production, re-work, loss of customers, tarnished reputation etc. The student should provide some practical examples of how product safety (for instance) might be affected (eg SimuLab’s analysis might show that the concentration of a particular additive was within the regulated food limits but was actually above the limits – this may put consumers at risk of getting sick). Teacher Guide PMLTEST506 Apply Spectrometric Techniques 2 Laboratory Operations Toolbox Teacher Guide Section: 1. Task: 2. Step: 1. Activity: Assignment- PPE Checklist Questions You have been to the Resources and Training Room to prepare the PPE checklist that will help you deliver the safety induction to SimuLab’s new trainee technician. Use the relevant SOP’s from the OHS Manual to prepare the checklist. Answers The checklist should be prepared after consulting the relevant Standard Operating Procedures (SOP’s) in SimuLab’s OHS Manual. These include: OHS11SOP: Safety Procedures – Reservoir Water Sampling OHS14SOP: Personal Protection Requirements – General Laboratory. Items for the checklist include: Reservoir Water Sampling PPE items Overalls Gumboots Latex gloves Life Jacket. General Laboratory PPE items Safety glasses with side shields Face-shields when handling hot or corrosive liquids or when carrying out reactions under vacuum Laboratory coats Substantial footwear (covered in) Hair net where hair is long Nitrile gloves when handling hazardous chemicals. Section: 1. Task: 3. Step: 2. Activity: Assignment - About ‘Chain of Custody’ Question A special system that monitors the flow and integrity of samples is called ‘chain of custody’ and is often used when test results need to stand up in a court of law. The tests might involve analysing a biological specimen for a drug of dependence or a commercial material where product substitution is alleged. 1. Search the World Wide Web for information on chain of custody so that you are able to describe (in your own words): an overview of what chain of custody means how it works and relates to the operation of a testing laboratory examples of its application Teacher Guide PMLTEST506 Apply Spectrometric Techniques 3 Laboratory Operations Toolbox Teacher Guide who fills out the form what information needs to be provided. 2. In addition to the above, obtain a copy of a suitable laboratory-based chain of custody form from the Internet. Put yourself in the position of a client of a laboratory wishing to submit the completed form together with its test sample. Use your imagination to fill out the form with general detail and also include the description of the sample that should reasonably indicate to a technician that something is wrong. The sample may have been mis-sampled, contaminated or have shown deterioration. The following key words, used alone or in combination, are suggestions to conduct your search on the Internet. ‘chain of custody’ laboratory contamination sampling guidance document Go to the Resources and Training Room to undertake your search (use the link provided on the main page and the computer marked Internet Search in the Room). Answer 1. In general, a chain of custody is a system, managed by use of an appropriate form, that controls the security and traceability of a sample during its time in a laboratory. The form could be prepared by the customer or by the testing laboratory. Chain of custody works by making people within the laboratory accountable for the sample at any particular time - the intention is to ensure that the sample is not removed, tampered with or altered in an undesirable way. Often chain of custody is implemented when a sample is subject to legal proceedings or other highly significant outcomes such as in commerce or sport. The use of a chain of custody form means that the laboratory needs to maintain control of the sample at all times, for example during testing and storage. To do this, a particular person or persons might need to be appointed to be with the sample during its time in the laboratory. If kept overnight, the sample might need to be placed under lock and key in a safe. Examples of samples/tests: Blood for alcohol analysis and paternity testing Urine for drugs of dependence in sport A sample of a commercial material that is alleged to have been substituted for an advertised superior quality product in the trade Environmental samples (eg river water or effluent) for heavy metals analysis (eg mercury or arsenic) In general, the people who fill out a chain of custody form are those who relinquish the sample (eg a customer or representative) and those who receive the sample (eg a representative of the laboratory). 2. For an example of a suitable form go to the home page of ‘Zymazusa’, an American environmental testing laboratory, and click on the ‘chain of custody’ link. Ensure that the student has made a good effort to fill out his/her form as much as Teacher Guide PMLTEST506 Apply Spectrometric Techniques 4 Laboratory Operations Toolbox Teacher Guide possible (including dates, number of containers received, analyses requested etc). The description of the sample needs to indicate that the sample is not what it is meant to be or has been affected in some way (eg contamination) as per Study Notes: Sample Description and Reporting. Section: 1. Task: 4. Step: 2. Activity: Assignment - Sample Preparation Procedures Question Locate two methods of spectroscopic analysis using a UV-visible spectrophotometer or an atomic absorption spectrometer and employing different sample preparation procedures. You are required to identify the sample preparation procedures involved in each method. These may be drawn from those discussed in Study Notes: More on Sample Preparation or others not specifically covered. Remember that there may be more than one procedure in each method. Describe and give detail of the test sample, the analyte, the procedural steps, chemicals and conditions for each method. Where you can, include the name of the particular procedure (eg acid digestion) and its purpose (eg dissolution of the analyte). Give the name of the methods and their sources. You can draw the methods from any appropriate source such as textbooks on analytical chemistry and from the Internet. If searching the Internet, the following key words are suggested (used alone or in combination): spectroscopy analysis ‘UV-Visible spectroscopy’ or ‘UV-Visible spectrophotometer’ AAS or ‘AA spectroscopy’ or ‘AA spectrometer’ Varian is a manufacturer of laboratory instrumentation and its web site contains a range of useful methods. Go to the Resources and Training Room to undertake your search (use the link provided on the main page and the computer marked Internet Search in the Room). Tutor Note Look for indications that the learner has applied sufficient research, thought and effort. Cross check the student’s responses against the methods they provide to determine whether each part of the activity has been addressed satisfactorily. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 5 Laboratory Operations Toolbox Teacher Guide Section: 2. Task: 1. Step: 1. Activity: Assignment – Astronomical Spectroscopy Question Explore the internet or suitable text books to find information on the use of spectroscopy in astronomy. Identify two examples of how spectroscopy has been used to determine chemical composition and physical or other properties/features of stars or other astronomical objects. Under the name of each object, list the chemical components identified, the particular properties/features discovered and any other relevant information. Where you can, identify the wavelengths produced by the spectograph that are associated with your listed items. Don’t forget to reference your sources including website URLs. The following key words are suggestions, used alone or in combination, to conduct your search on the internet. spectroscopy astronomy spectrograph Go to the Resources and Training Room to undertake your search (use the link provided on the main page and the computer marked Internet Search in the Room. Tutor Note Look for indications that the learner has applied sufficient research, thought and effort. You could cross check the student’s responses against the sources they provide. Section: 2. Task: 3. Step: 6. Activity: Assignment – How Light Behaves Questions Based on the study notes within the task, How Light Behaves, prepare written answers to the following questions. 1. What does opaque mean? 2. Why is light intensity less as you move away from a light bulb? 3. What type of reflection is obtained from a smooth surface: Teacher Guide PMLTEST506 Apply Spectrometric Techniques 6 Laboratory Operations Toolbox Teacher Guide Specular? Diffuse 4. If a light ray strikes a polished metal surface at a 60o angle, what will be the angle of reflection of the light ray? 5. What is lateral inversion? 6. The diagram shows a parallel beam of light striking a concave mirror. One of the reflected rays has been drawn for you. Draw the other three reflected rays. 7. A sketch of a beam of light passing from air into a slab of glass is shown. Which of the light rays (A, B, C or D) best shows the path of the refracted light ray in the glass? 8. What happens when the angle of incidence of a light ray travelling from glass into air is: greater than the critical angle? less than the critical angle? 9. Draw a diagram to show how a prism can be used to change the direction of a light ray by 90o. 10 When a prism is used to separate white light into its component colours, which component colour of the visible spectrum is: refracted least? refracted most? 11. What is a diffraction grating? 12. What is a diffraction grating used for? 13. Which of the following lenses will focus light? Convex Concave 14. What is chromatic aberration and how is it corrected for a lens? Answers 1. A material is one that blocks out light or that light does not pass through. 2. Light intensity is less as you move away from a light bulb because the light rays spread out from the source. 3. Specular reflection is obtained from a smooth surface. 4. 60° 5. Lateral inversion occurs with a mirror when the left side of an object appears as the right side in its image. 6. The three remaining rays intersect (and form the focus) at the mid point of the drawn ray between the middle two incident beams. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 7 Laboratory Operations Toolbox Teacher Guide 7. Ray C best shows the path of the refracted light (air to glass refracts towards the normal). 8. When the angle of incidence of a light ray travelling from glass into air is greater than the critical angle, total internal reflection occurs (the light does not leave the glass). When the angle is less than the critical angle the light ray is refracted out of the glass into air. 9. Refer to the diagram in Study Notes: Prisms. 10. When a prism is used to separate white light into its component colours, red light is refracted least and violet light the most. 11. A diffraction grating has a polished metal surface or a glass surface on which there are a large number of very closely spaced grooves. 12. Diffraction gratings are used to separate light into its component wavelengths. 13. A concave mirror will focus light. 14. Chromatic aberration occurs when the different colours in white light are refracted by a lens different amounts. The image will appear to have coloured edges and is not clear. One method of preventing this defect is to place another lens in the light path to combine the colours again. Section: 2. Task: 5. Step: 2. Activity: Assignment – Here’s to Beer’s Law Questions Based on the study notes within the task, Here’s to Beer’s law, prepare written answers to the following questions showing your workings where appropriate. 1) Nickel sulfate solution is green-blue in colour. Which of the following wavelengths would be most strongly absorbed by nickel sulfate solution? 430 nm 490 nm 530 nm 590 nm 2) For absorption analysis, why do we choose a wavelength of incident light that is strongly absorbed by the solution? 3) A KMnO4 solution appears purple (violet) to the naked eye. (a)What colour (mainly) is absorbed by the solution? (b) The wavelength of maximum absorbance is closest to: 400 nm 500 nm Teacher Guide PMLTEST506 Apply Spectrometric Techniques 8 Laboratory Operations Toolbox Teacher Guide 600 nm 700 nm (c ) A filter to be used in the colorimetric analysis of KMnO4 solution should transmit mainly: purple blue green yellow. 4) Standard solutions of X2+ with molar concentrations of 0.16, 0.14, 0.12, 0.10 and 0.080 M were analyzed at 420 nm in 0.50 cm cells. Their respective absorbances were found to be 0.80, 0.70, 0.60, 0.50 and 0.40. (a) Another solution of X2+ gave an absorbance reading of 0.525. What is its concentration? Hint: prepare a calibration graph. (b)Under these conditions, what is the value of for X2+? Hint: consider a point on the calibration curve. (c)What absorbance would you expect for 0.20 M X2+ in a 0.50 cm cell? What transmittance is this equal to? (d) At 420 nm, what concentration of X2+ would give an absorbance reading of 0.10 in a 1.0 cm cell? (e) A 10.00 mL aliquot of drain water was diluted to 200.0 mL before analysis at 420 nm in a 2.0 cm cell. If the absorbance reading of 0.60 is due only to the presence of X2+, calculate the concentration of X2+ in the drain water. 5) The intensity of the colour of a solution of unknown concentration is visually compared to a set of standards. The intensity of the unknown appears to be lighter than the 16 mg/L standard and darker than the 12 mg/L standard. (a)Estimate the concentration of the unknown. (b)What are the main limitations of the visual standard series method of colorimetry? 6) The intensity of a Ni2+ sample solution was a lighter colour than a 40.0 mg/L standard solution of Ni2+. A good visual colour match was obtained after diluting 6.0 mL of the standard to 50.0 mL. What was the concentration of the Ni2+ in the original sample? Answers 1. 590nm (refer to table in Study Notes: Beer’s Law) 2. Strong absorption means that Beers Law can be applied to determine the concentration of the absorbing chemical species at the particular wavelength. 3. (a) Green (refer to table in Study Notes: Beer’s Law) (b) 500 nm (refer to table in Study Notes: Beer’s Law) (c ) Green (refer to table in Study Notes: Beer’s Law) – a green filter will transmit green light which is the colour that will be absorbed by the KMn04 solution. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 9 Laboratory Operations Toolbox Teacher Guide 4. (a) A calibration graph of concentration (x axis) versus absorbance (y axis) is prepared and is then used to determine concentration at the nominated absorbance. The required answer is 0.105 M. (b) Choose any point on the calibration graph and determine the matching absorbance and concentration values (could use those in 3b above). Transposing Beer’s law and substituting these values gives an extinction coefficient () of 10. (c ) Assuming that Beer’s law is obeyed at the higher concentration (ie in the range beyond the calibration graph prepared), Beer’s law can be used ( where = 10 mol-1 L cm-1, l = 0.5 cm and c = 0.20 mol L-1) to give an absorbance of 1.0 (at 420 nm). This is equal to a transmittance of 10%. (d) Assuming that Beers Law is obeyed at low absorbances, Beer’s law can be used to give a concentration of 0.010 M (where = 10 mol-1 L cm-1, l = 1.0 cm and A = 0.10). (e) The concentration in the diluted sample is 0.03 M (transpose Beer’s law so that concentration is the subject and use = 10 mol-1 L cm-1, l = 2.0 cm and A = 0.60). The concentration of the original drain water is 20 times that of the diluted sample (ie 0.60 M). 5. (a) The estimated concentration of the unknown is 14 mg/L. (b) The main limitation of the visual method is that it is an estimation based on personal interpretation of the relative intensity of the colours of the sample and standards. In this respect the method suffers from inaccuracy compared to instrumental techniques. The method may also be time consuming. 6. The sample concentration is equal to the final concentration of the standard ie 4.8 mg/L. Section: 3. Task: 1. Step: 1. Activity: Assignment – The Origin UV-Vis Spectra Questions Building on the content in Study Notes: Origin of UV-Vis Spectra, prepare brief written answers to the following questions. You will need to consult textbooks, search the internet or use other sources to find the required answers. 1. An excited molecule can return to its ground state by giving up its absorbed energy. Fluorescence and photochemical decomposition are two modes through relaxation can occur. a. Explain what fluorescence is and how it occurs. b. Fluorescence is the basis of an analytical chemistry technique. Compare its performance to absorption based methods. For what samples is fluorescence analysis commonly used? Teacher Guide PMLTEST506 Apply Spectrometric Techniques 10 Laboratory Operations Toolbox Teacher Guide c. What is photochemical decomposition and how might it be a problem in taking absorbance readings in spectroscopic analysis? 2. UV-Visible spectroscopy primarily concerns the study of electronic transitions. What branch of spectroscopy concerns vibrational and rotational transitions? Don’t forget to reference your sources including website URL’s. Remember to include a copy of the material for your tutor. Answers 1. a. Fluorescence is an important emission process in which atoms or molecules are excited by absorption of a beam of electromagnetic. The excited species then relax to the ground state giving up their excess energy as photons (rather than as heat). This is referred to as radiant emission. Fluorescence takes place very rapidly is generally complete in 10-5 seconds or less. b. Fluorescence methods are generally one to three orders of magnitude more sensitive than absorption methods. Sensitivity is enhanced by increasing the power of the excitation beam or amplifying the detector signal (neither of which are an option for absorption methods). Fluoroemetry is applied for the analysis of food products, pharmaceuticals, clinical samples and natural products. c. Photochemical decomposition occurs when a chemical species absorbs electromagnetic radiation to form an excited state. The excited species then relaxes by forming a new species (rather than maintaining the existing species as normally occurs with absorption and emission spectroscopy). Photochemical decomposition could occur during UV-Vis absorption analysis whilst the analyte was exposed to the radiation. This could lead to unexpected absorbance/transmission results. 2. Infra red spectroscopy Section: 3. Task: 1. Step: 3. Activity: Assignment – How Organic Compounds Absorb UV-Vis Radiation Questions This activity is based on Study Notes: How Organic Compounds Absorb UV-Vis Radiation. You will need to search the internet, appropriate textbooks or other sources to prepare answers to the following questions. 1. Obtain a copy of a table that shows absorption characteristics (max and, if available, max ) of common organic chromophores. Highlight those chromophores that might be of practical use to a spectroscopy analyst. Explain the reason for your choices. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 11 Laboratory Operations Toolbox Teacher Guide 2. What is a red shift (or bathochromic effect) and how is it produced? Include an explanation of why you think a ‘red shift’ is so named. 3. What is a blue shift (or hypsochromic effect) and how is it produced? Include an explanation of why you think a ‘blue shift’ is so named. 4. Provide an example, with supporting detail, of compounds where increasing the number of double bonds in conjugation increases max for the respective absorption peak(s). Show the impact on max values. Simple conjugated polyenes is one example of the type of compounds you could study. 5. What are the technical difficulties involved with trying measuring ultraviolet absorption below 200 nm? Send your findings and a copy of your referenced source materials from this activity to your tutor. Answers 1. Such tables can be found in various chemistry text books. The student is expected to highlight chromophores that exhibit max above 200 nm where spectroscopic measurements are of most practical use. It is technically difficult to measure absorbances below this wavelength. 2. A red shift (or bathochromic effect) is a shift of an absorption maximum towards a longer wavelength (as it were, towards the red end of the visible spectrum). It may be produced by a change of solvent or by the presence of a substituent on a chromophore which leads to a red shift. For example, the conjugation of the lone pair on the nitrogen atom of enamine shifts max from the isolated double bond value of 190 nm to about 230 nm (the chromophore is extended to give a new chromophore). 3. A blue shift (or hypsochromic effect) is a shift towards a shorter wavelength (as it were, towards the blue end of the visible spectrum). This may be caused by a change in solvent and also by such phenomena as the removal of conjugation. For example, the conjugation of the lone pair of electrons on the nitrogen atom of aniline with the π bond system of the benzene ring is removed on protonation. Aniline absorbs at 230 nm but in acid solution the main peak is almost identical with that of benzene being now 203 nm. 4. Textbooks etc should contain various examples for the student to present. Check that the response indicates an understanding of the concept. 5. The technical difficulties associated with trying to measure absorption below 200 nm include absorption by the atmosphere (measurements therefore need to be made in an evacuated spectrophotometer hence 120 to 200 nm is known as the vacuum ultraviolet range). Quartz also absorbs below 200 nm hence lithium fluoride optics need to be employed. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 12 Laboratory Operations Toolbox Teacher Guide Section: 3. Task: 1. Step: 4. Activity: Assignment – Absorption of UV-Vis Radiation by Transition Metal Complexes. Questions The questions that follow are based on Study Notes: About Transition Metal Complexes and Study Notes: Absorption of UV-Vis Radiation by transition Metal Complexes. You will need to search the internet, consult appropriate textbooks or other sources to answer the questions. You may find useful the websites that have been provided on transition metal complexes. 1. What are the colours of the hexa-aqua transition metal ions (in aqueous solution) for chromium, iron, cobalt, nickel and copper. 2. Explain why the complexes of the transition metals scandium and zinc are colourless (hint: investigate the electronic structure of the respective ions ie Sc3+and Zn2+). 3. Provide an example of each to demonstrate how the colour of a transition metal complex in solution changes with oxidation state and the ligand. 4. Charge transfer is a type of electronic transition that can occur in particular transition metal complexes. Describe the nature of such transitions, the overall intensity of absorption (use max values if available), the region(s) in which absorption typically occurs and provide several examples of complexes that display this behaviour (where available, submit copies of spectra marked to show the absorption). Comment on the suitability of such metal complexes to quantitative analysis. Remember to include a copy of the reference materials for your tutor. Answers 1. The colours of the complexes are displayed in the Chemguide site (WWW catalogue). They are as follows (or similar): chromium-dark blue, iron-light blue/violet, cobalt-red, nickel-green, copper-sky blue. 2. Although scandium is a member of the d block, its ion (Sc3+) hasn't got any d electrons left to move around. Scandium(III) complexes are therefore colourless because no visible light is absorbed. In the zinc case, the 3d level is completely full - there aren't any gaps to promote an electron into. Hence zinc complexes are also colourless. 3. Examples can be found in text books and other sources. From the Chemguide site, change in oxidation state >> [Cr(H2O)6]2+ is sky blue while [Cr(H2O)6]3+ is violet/blue/grey colour; re ligand > [Cu(H2O)6]2+ is sky blue while [Cu(NH3)4(H2O)2]2+ is dark blue. 4. Charge transfer absorption in transition metal complexes involves the transfer of an electron from the ligand to the metal complex or vice-versa. This is a type of internal oxidation/reduction process in which the metal ion can be either oxidised or reduced. Charge transfer transitions are usually intense (max ~ 104) which means that the complexes are well suited to quantitative analysis (good sensitivity). Absorption normally occurs in the UV region but may extend into the visible region. Examples are numerous and include permanganate ions and iridium-pyridine complex. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 13 Laboratory Operations Toolbox Teacher Guide Section: 3. Task: 2. Step: 1. Activity: Activity- UV-Vis Monochromator Questions The questions that follow are based on Study Notes: UV-Vis Monochromators. You will need to search the internet, consult appropriate text books or other sources to answer the questions. 1. Find an example of a series of scans that illustrates the effect of slit width (bandwidth) on spectral detail. Ensure each scan is labelled with the respective slit width. Describe the detail that you see to distinguish between the individual scans. 2. Colorimeters and photometers use coloured glass filters. What function do filters perform? Compare and contrast with monochromators. To use the internet, go to the Resources and Training Room and click on a search engine provided or use one of your own choice. Remember to include a copy of the reference materials for your tutor. Answers 1. Look for an answer that shows thought and research effort. Skoog/Leary (Instrumental Analysis) Figure 7-10 displays a series of spectra demonstrating the effect of bandwidth on spectral detail. The essential feature is that as bandwidth decreases the spectral detail becomes sharper – sharp peaks become evident. 2. Look for an answer that shows thought and research effort. In essence, coloured glass filters transmit a particular colour comprising a relatively broad range of wavelengths and are thus polychromatic. Monochromators, on the other hand, transmit light of a very narrow wavelength range. Section: 3. Task: 2. Step: 1. Activity: Assignment - UV-Vis Detector Questions The questions that follows are based on Study Notes: UV-Vis Detector. You will need to search the internet, consult appropriate text books or other sources to answer the questions. Diode array detectors have become increasingly important in the modern spectrophotometers. 1. Investigate and report on this technology taking particular note to describe the basis of its operation and its superior performance characteristics compared to other detector types. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 14 Laboratory Operations Toolbox Teacher Guide 2. Submit a copy of a picture or a schematic drawing of the basic components of a spectrophotometer with a diode array detector – describe how and why the layout differs from that of the instrument shown in the Study Notes. To use the internet, go to the Resources and Training Room and click on a search engine provided or use one of your own choice. Remember to include a copy of the reference materials for your tutor. Answers 1. Look for an answer that shows thought and research effort. Descriptions of the operating principle of diode array detectors can be found in text books and other sources. The superior performance of this type of detector is attributed to it being able to measure absorption at all wavelengths at high speed (no need to renew the blank solution). Because these are solid state devices they have high reliability. 2. Look for an answer that shows thought and research effort. The layout of a spectrophotometer employing a diode array detector would show polychromatic light from the source passing directly through the sample and then passing through the dispersion device whereupon all transmitted wavelengths are separated prior to striking the detector. With the spectrophotometer shown in the Study Note, monochromatic light passes through the sample and then onto the detector. In effect then, the use of a diode array detector transfers the sample cell to the front end of the optical system. Section: 3. Task: 3. Step: 2. Activity: Assignment - Analytical Wavelength. Questions This activity is based on Study Notes: Analytical Wavelength. 1. Draw a diagram of concentration versus absorbance showing two (straight) calibration lines for an imagined analyte. The lines represent analytical wavelengths taken from different points on the analyte’s spectrum, possibly max and half max (note that both lines pass through the origin). You are told that the lowest reliable absorbance that is able to be read for this particular method is 0.1. In general terms (using concentration values if possible), demonstrate and describe on the graph the impact of this absorbance restriction on the sensitivity of the method at the two wavelengths. 2. Draw an imagined spectrum showing a single peak having a plateau (ie max) at an absorbance of 1.0 (make the peak about 150 mm high). The valleys on either side of the peak are 100 nm apart (draw about 200 mm from each other). From your spectrum, do calculations to estimate the maximum variation in absorbance at max ± 2 nm and at max ± 5 nm. Do the same calculations for a wavelength at a point about half way up the peak. Compare and contrast the results. What do they indicate about the precision of the results obtained with max as the analytical wavelength. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 15 Laboratory Operations Toolbox Teacher Guide Answers 1. Look for thought and effort in this answer. The graph would show 2 lines of different slope and both would pass through the origin as specified. The smaller slope line is attributed to absorbance readings taken with the analytical wavelength at a non-max value while the line with the larger slope is attributed to max. Drawing a horizontal line across the graph at 0.1 absorbance should show that the variation in minimum concentration able to be detected at both wavelengths. The higher concentration will be determined with max. 2. Look for thought and effort in this answer. The answer should clearly demonstrate that significantly better precision (ie narrower range of values) is obtained when using max as the analytical wavelength (a clue is the last graphic in the Study Note). It would be interesting to note the student’s observations on the comparison of precision at max ± 5 nm and at non-max ± 2 nm Section: 3. Task: 3. Step: 3. Activity: Assignment – Deviations from Beers Law Questions The questions that follow are based on Study Notes: Deviations from Beers Law. You will need to search the internet, consult appropriate text books or other sources to answer the questions. 1. The level of stray radiation (or stray light) is an important performance characteristic of a spectrophotometer. What is stray radiation and how is it generated in a spectrophotometer? How does stray radiation impact on the linearity of Beers Law? Find a drawing that demonstrates the effect and describe what you see. If linearity is assumed when stray light is actually present, what is the general impact on the value of the calculated concentration? 2. If a solution gave an absorbance reading that was above the linear range for Beers Law, what two measures might the analyst perform to bring the solution into range? (Hint: consider the Beers Law variables). 3. Fe(SCN)2+ has a molar absorptivity of 4870 at 455 nm. Calculate the molar concentration range of standard solutions of Fe(SCN)2+ that would be within the acceptable absorbance limits of 0.1 to 0.8 using a 1 cm cell. To use the internet, go to the Resources and Training Room and click on a search engine provided or use one of your own choice. Remember to include a copy of the reference materials for your tutor. Answers 1. Look for some thought and research effort in the answer. Stray radiation is defined as detected light of any wavelength that lies outside the bandwidth of the selected wavelength. It occurs to some extent depending upon spectrophotometer design and arises from instrumental imperfections due to light scattered off the surfaces of prisms, lenses, filters and windows. Stray radiation often differs markedly in wavelength from the principal radiation and may not have passed through the sample or solvent. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 16 Laboratory Operations Toolbox Teacher Guide At high absorption levels, stray light causes a negative bias in instrument response and eventually is the limiting factor for absorbance, and thereby concentration, that can be measured (low results occur). The photometric accuracy (ie the accuracy of absorbance readings) of the instrument is thus compromised. A graph demonstrating the effect of stray radiation (available in text books) would show the calibration curve deviating under the straight line at high absorbances. 2. The two measures are to dilute the sample solution into the required absorbance range or to use a sample cell with a shorter optical path length such that absorbance fell within range. 3. At A = 0.1, c = A/( x l) = 0.1/(4870 x 1.00) = 2.05 x 10-5 M At A = 0.8, c = A/( x l) = 0.8/(4870 x 1.00) = 1.64 x 10-4 M Section: 3. Task: 4. Step: 1. Activity: Assignment – Virtual Spectrophotometer Questions This activity is based on the outcomes of the simulated analysis of NoSlime using the virtual spectrophotometer. Answer the questions posed below. Algicide Concentration 1. Prepare a calibration curve for the algicide product (draw to scale and label fully). 2. What is the concentration of algicide in the concentrated product? Express the result to the nearest g/L. General Questions 1. What can you say about the linearity of the calibration curve and the method’s adherence to Beers Law? 2. What is the purpose of waiting 30 minutes before absorbance readings are taken? 3. Why was 5 nm selected as the slit width rather than a narrower value such as 0.1 nm? 4. The use of the sample cell in the simulation is simplified. In actual practice, what would be done between each reading? 5. Imagine that one of the standards gave a very high absorbance reading that was well out of step with the values for the other standards. What are the possible explanations for this observation? 6. What might you troubleshoot the problem to be if the spectrophotometer’s display went blank at some stage during the analysis and stayed that way? Assume that the instrument remained powered up. Send your answers together with the calibration curve and all calculations to your tutor. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 17 Laboratory Operations Toolbox Teacher Guide Answers Algicide Concentration 1. The student should present a graph drawn to scale and fully labelled as requested. The use of Excel graphing capabiliy would be a good approach otherwise a manually prepared graph is quite satisfactory. 2. The absorbance of 0.666 provided by the virtual spectrophotometer should correspond on the calibration graph to a concentration of 1.2 g/L in the prepared solution of SL2003/455 (depending upon how well the calibration curve is fitted to the data points, 1.1 to 1.3 g/L would be acceptable). Allowing for dilution (75 times), the concentration of algicide in the concentrated product is 90 g/L (90± 2 g/L would be acceptable). General Questions 1. The calibration curve shows good linearity and therefore adherence to Beers Law over the range employed (R2 = 0.99). 2. The 30 minute wait is to allow the spectrophotometer components to warm up (particularly with regard to the source lamps and detector). 3. A wide slit width is chosen for quantitative work since it provides a relatively broad absorption peak and therefore small errors in setting the analytical wavelength (ie max) have a minimal impact on absorbance and therefore on the accuracy/precision of the calculated concentration. The use of a narrow slit width may provide a sharper peak which may be less tolerant to errors in wavelength setting. 4. In practice, the sample cell would be rinsed with each solution and have its optical face wiped dry with lint-free tissue prior to reading in the spectrophotometer. 5. A very high reading could suggest that the sample cell was placed in the spectrophotometer with the non-optical (frosted) face towards the source light beam rather than the other way round. It is important that the student identify this as the possible answer however there could be other suggestions as well eg the wrong solution was measured. 6. The classic troubleshooting approach with a spectrophotometer when something like this occurs is to check the status of the source lamps. These items do not last forever and occasionally need to be replaced because they breakdown or lose intensity. Section: 3. Task: 5. Step: 1. Activity: Assignment – Reporting Results Questions Based on the requirements in Test Result Reporting Procedure prepare a suitable proforma for a Laboratory Test Sheet that might be used by SimuLab. Showing all required sample details/results and customer details, fill out a copy of the prepared Laboratory Test Sheet for the analysis of NoSlime. Make up any details that are not provided. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 18 Laboratory Operations Toolbox Teacher Guide Answers Ensure that the student presents a completed Laboratory Test Sheets that is clearly formatted and addresses each of the criteria listed in the Test Result Reporting Procedure. Look for the name of the customer provided in Task 4 (SimuTown Water Treatment Pty Ltd). Also check that the test result has been correctly recorded including the units. Section: 4. Task: 1. Step: 1. Activity: Assignment - The Origin AAS Spectra You will need to participate in a discussion forum in order to answer the questions below. Go to the Discussion Forum: The Origin of AAS Spectra to discuss this AAS topic with specific relevance to the questions posed below: 1. In AAS where do the adsorption and emission spectra arise from? 2. What are the differences between the two spectra? 3. Why is adsorption rather than emission usually used for analysis? You are expected to contribute at least three times to the forum. Use the button marked Forum on the main page. Send your answers to your tutor along with a printout of the discussion forum in which you have participated at least three times. Answers Students may give more comprehensive answers that the minimum listed below and they may include photographs of simple AAS and AES spectra. 1. In AAS where do the adsorption and emission spectra arise from? Adsorption spectra arise from adsorption of the light at a particular wavelength (energy) that corresponds to the quanta involved in an outer shell electron becoming excited to a higher energy orbital. Emission spectra arise when the electron drops back to its normal position (atom is said to be in its ground state) and light of a wavelength corresponding to the difference in energy is emitted by the atom. 2. What are the differences between the two spectra? AAS is more sensitive than Atomic Emission Spectroscopy (AES) AAS leads to a reduction in the light travelling through the flame. AES leads to an increase in emitted light. 3. Why is adsorption rather than emission usually used for analysis? More atoms in the ground state. Freedom from spectral interferences. Atoms of other elements do not adsorb the specific wavelength. Simple spectra produced that only require a simple monochromator. Number of ground state atoms is relatively temperature dependent. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 19 Laboratory Operations Toolbox Teacher Guide Section: 4. Task: 1. Step: 2. Activity: Assignment – The Application of AAS Questions The questions that follow are based on Study Notes: The Application of AAS. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. The atomic absorption line is somewhat broader than the incident emission line from the spectrophotometer’s lamp. This is attributed to two effects known as Doppler Broadening and Pressure Broadening. Describe how these effects cause absorption line broadening in AAS. Do not forget to reference your sources including website URLs. Remember to include a copy of the reference materials for your tutor. To use the Internet, go to the Resources and Training Room and click on a search engine provided or use one of your own choice. Send your findings and a copy of your referenced source materials from this activity to your tutor. Answers Doppler broadening results from the rapid motion of atoms as they emit or absorb radiation. Atoms moving towards thew detector emit wavelengths that are slightly shorter than wavelengths emitted by atoms moving at right angles to the detector. This is called the Doppler Shift. The effect is reversed for atoms moving from the detector. The most important effect for AAS is that the Doppler Effect cause broadening of the absorption lines and this increase increases as the flame temperature increases. Pressure broadening comes from collisions between atoms that result in slight changes in their ground-state energies and slight energy differences between their ground and excited states. This effect also increases with temperature. Thus broader absorption peaks are always found at elevated temperatures. Section: 4. Task: 1. Step: 2.: Activity: Assignment – General Aspects of AAS Analysis Questions The questions that follow are based on Study Notes: General Aspects of AAS Analysis. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. 1. Obtain a copy of the Periodic Table and mark the 67 elements that can be measured using AAS. 2. List examples of sample materials than can be measured using AAS. Be sure to include examples of environmental, geological and biological samples. 3. What wavelength would you use to measure the following metals using AAS: Copper Nickel Teacher Guide PMLTEST506 Apply Spectrometric Techniques 20 Laboratory Operations Toolbox Teacher Guide 4. Describe the method used for the dissolution of a specified sample. You specify the sample. Do not forget to reference your sources including website URLs. Remember to include a copy of the reference materials for your tutor. To use the Internet, go to the Resources and Training Room and click on a search engine provided or use one of your own choice. Send your findings and a copy of your referenced source materials from this activity to your tutor. Answers 1. Obtain a copy of the Periodic Table and mark the 67 elements that can be measured using AAS. The periodic table showing the relevant elements highlighted. Thus information was given and is used here to reinforce the material. Students should be able to reproduce this exactly without errors. 2. List examples of sample matrices than can be measured using AAS. Be sure to include examples of environmental, geological and biological samples. Environmental – Waste Water, Ground Water Geological – ores, minerals Biological – blood, urine, serum 3. What wavelength would you use to measure the following metals using AAS: Copper – 324.7 nm, Nickel – More difficult but around 232.0 nm. 4. Describe the method used for the dissolution of a specified sample. You specify the sample. This could be anything. Look for answers that include the concepts of dilution, dissolution using acids such as nitric acid, low remaining solids. Section: 4. Task: 2. Step: 1. Activity: Assignment – AAS Light Source Questions The questions that follow are based on information available on the internet. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. To use the Internet, go to the Resources and Training Room and click on a search engine provided or use one of your own choice. A good place to start is: www.varianinc.com (Type ‘Hollow Cathode Lamp into the ‘Search’ window then click on ‘Responses to Hollow cathode Lamp FAQs’) 1. What do Hollow Cathode Lamps look like? Provide a picture of a lamp. 2. What is a ‘getter spot’? Teacher Guide PMLTEST506 Apply Spectrometric Techniques 21 Laboratory Operations Toolbox Teacher Guide 3. How is the lamp treated/aged/settled in before it leaves the factory? 4. What is the purpose of the fill gas in the lamp? 5. Are lamps single element or multi-element? Explain why and the benefits associated with their use. 6. Give one example of spectral interferences. 7. Why do lamps wear out and how do we know when this happens? Send your answers to your tutor along with a printout of any Internet site materials used in this activity. Answers Students may give more comprehensive answers that the minimum listed below and they may include photographs of simple AAS and AES spectra. 1. What do Hollow Cathode Lamps look like? Provide a picture of a lamp. Student should provide a photograph of an HCL. 2. What is a ‘getter spot’? A zirconium metal film deposited on the inside of the HCL near the anode. It is highly reactive and acts as a scavenger of traces of oxygen and other impurity gases. It increases the life of the lamp. 3. How is the lamp treated/aged/settled in before it leaves the factory? Aged to ensure lamp is ready for immediate use, tested to ensure it meets requirements for intensity and stability. 4. What is the purpose of the fill gas in the lamp? Allows the sputtering of the cathode material. The gas is ionised due to a high voltage and the ions hit the cathode surface knocking metal atoms loose. The atoms are further excited then relax back to their ground state emitting radiation characteristic of that element. 5. Are lamps single element or multi-element? Explain why and the benefits associated with their use. Both are available with multi-element having up to six elements in the cathode and provide convenience of many elements in one lamp. 6. Give one example of spectral interferences. Weak silver ion line close to Pb 217.0 nm in Ag/Cd/Pb/Zn multi-element lamps. There are others. 7. Why do lamps wear out and how do we know when this happens? Physical damage due to rough handling Gas fill absorbed into the internal surfaces of the lamp Operating lamp at excessive lamp currents leading to cathode overheating and damage. Evidenced by erratic cathode discharge or complete failure to ‘strike’. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 22 Laboratory Operations Toolbox Teacher Guide Section: 4. Task: 2. Step: 2. Activity: Assignment – Flame Atomiser Questions You will need to participate in a discussion forum in order to answer the questions below. Go to the Discussion Forum: Flame Atomiser to discuss this AAS topic with specific relevance to the assignment posed below. You are expected to contribute at least three times to the forum (once to submit your proposal and twice to analyse critically the proposals of two other participants). Use the button marked Forum on the main page. Send your proposal to your tutor along with a printout of the discussion forum in which you have participated a further two times. The questions that follow are based on information available on the internet. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. To use the Internet, go to the Resources and Training Room and click on a search engine provided or use one of your own choice. A good place to start is: www.varianinc.com 1. Select one commercially available flame AAS. 2. Pretend that you are a new sales representative for this company and your boss has asked you to develop a sales proposal for one of your larger customers who is thinking of purchasing this AAS and is particularly interested in your burnernebuliser system. In your proposal concentrate on the burner-nebuliser system only. 3. Find out as much information as you can about the burner-nebuliser system for this AAS including: a. Advantages b. Disadvantages, and c. Troubleshooting. 4. Access one ‘application’ for this AAS. An application is a method for analysis of a specified analyte. This will be the analysis that your customer wants to use. 5. Develop and present your sales proposal to the Discussion Group and ask for their comments on your proposal. 6. You should comment on two other proposals. Send your proposal including comments from two other Forum members and your comments on two other proposals to your tutor. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 23 Laboratory Operations Toolbox Teacher Guide Answers There are many possible answers and AAS systems that may be used in this assignment but it would be reasonable to suspect that students would often opt for Varian AAS systems. Look for an answer that: covers all the points listed above demonstrates an in-depth knowledge of the burner-nebuliser system demonstrates a degree of effort provides a good summary in the ‘Sales Proposal’ – good students may well prepare a PowerPoint or similar presentation analyses the responses to their proposal from other Forum members shows some critical analysis of two other proposals. Section: 4. Task: 2. Step: 2. Activity: Assignment – Gas Mixtures Questions The questions that follow are based on information available on the internet. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. 1. From the following list of elements, which would you use air/acetylene, nitrous oxide/acetylene or either gas mixture for analysis? Au, Be, Cr, Dy, Fe, Ho, In, Lu, Mg, Os, Rh, Tm and Yb. What are the advantages of using hotter flames? 2. What are the disadvantages of using hotter flames? 3. Interferences for Mg, Ca, Sr and Ba in the presence of Al, Si and P are particularly severe due to the formation of refractory aluminates, silicates and phosphates in the flame. This interferes with the production of analyte atoms. Describe two ways in which this may be prevented or overcome. 4. In a lean flame is the oxidant or the fuel in excess? Send your answers to your tutor along with a printout of any Internet sites materials used. Answers Students may give more comprehensive answers that the minimum listed below. 1. From the following list of elements, which would you use air/acetylene, nitrous oxide/acetylene of both gas mixtures for analysis? Au, Be, Cr, Dy, Fe, Ho, In, Lu, Mg, Os, Rh, Tm and Yb. Air/acetylene Au Fe In Rh Nitrous oxide/acetylene Be Dy Ho Lu Tm Yb Teacher Guide PMLTEST506 Apply Spectrometric Techniques Either Cr Mg Os 24 Laboratory Operations Toolbox Teacher Guide Note: There may be slight differences due to text/site consulted. What are the advantages of using hotter flames? Aids decomposition of recalcitrant species such as oxides Increases number of free atoms Increases signal Avoids interferences that may arise in cooler flames. 2. What are the disadvantages of using hotter flames? Temperature may not always be the main decomposition agency and increasing flame temperature will not solve all analytical problems. Expense. 3. Interferences for Mg, Ca, Sr and Ba in the presence of Al, Si and P are particularly severe due to the formation of refractory aluminates, silicates and phosphates in the flame. This interferes with the production of analyte atoms. Describe two ways in which this may be prevented or overcome. Any two of the following will suffice. Use of a nitrous oxide/acetylene flame Use of a buffer element that is added to compete with the analyte element for attachment of the interfering group leading to complete atomisation at lower temperatures Match standard solutions to the sample carefully with respect to the interfering element but this is not always possible as it demands knowledge of sample composition. 4. In a lean flame is the oxidant or the fuel in excess? Lean flame is oxidant rich – fuel poor Rich flame is oxidant poor – fuel rich. Section: 4. Task: 2. Step: 2. Activity: Assignment – Positioning the Burner Questions The questions that follow are based on information available on the internet. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. 1. Find a picture of a commercially available AAS. 2. Indicate on the AAS where the burner position controls are. A close up picture or diagram would be useful. 3. Find a picture or a drawing of the burner from this AAS. 4. Indicate how the operation of the controls affects the position of the burner. 5. Find information of two problems that may affect the operation of the burner and how to overcome these problems (troubleshooting). Teacher Guide PMLTEST506 Apply Spectrometric Techniques 25 Laboratory Operations Toolbox Teacher Guide Send your answers to your tutor along with a printout of any Internet sites materials used. Answers This is a simple and straightforward assignment. 1. Find a picture of a commercially available AAS. Any AAS will do as long as it is flame, shows the position of the burner controls and the burner itself. This may require a number of separate pictures or diagrams. 2. Indicate on the AAS where the burner position controls are. A close up picture or diagram would be useful. Self explanatory but the student should indicate what each of the controls does. 3. Find a picture or a drawing of the burner from this AAS. Self explanatory. 4. Indicate how the operation of the controls affects the position of the burner. For example, the student should clearly show that moving control ‘X” in a clockwise fashion, causes the burner to move in a certain direction. 5. Find information of two problems that may affect the operation of the burner and how to overcome these problems (troubleshooting). Examples include: Blocking of burner vent with ash and residue. Run at a higher temperature, careful with sample preparation, clean more regularly. Asymmetric flame – again may be due to blocking. Poor sample flow rates. Block in sample tubing, problem with nebuliser. Flame temperature drops – poor nebulisation leading to larger sample droplets Flickering flame – gas flow problem. Section: 4. Task: 2. Step: 3. Activity: Assignment – Graphite Furnace Atomiser Questions The questions that follow are based on information available on the internet. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. 1. Source a commercially available GFAA. 2. Give full details of make, model and a photograph of the equipment. 3. List and describe the important features of this GFAA according to the manufacturer’s information. 4. Obtain a schematic diagram of the components of this GFAA or a similar GFAA. Label the major components on this diagram and describe briefly what each Teacher Guide PMLTEST506 Apply Spectrometric Techniques 26 Laboratory Operations Toolbox Teacher Guide component does. Note: at this stage you have not covered all components of AAS equipment in detail. You should consider this question as a preview of what is to come in further sections of this section. 5. Find and detail one application for this GFAA. An application is essentially an SOP developed by the manufacturer describing how to use their equipment to measure a specified analyte. 6. What are the important/crucial steps in this application and why? Do not just list them all. 7. Give three reasons why GFAA is better than flame AAS? Send your answers to your tutor along with a copy of any materials that you have used for this assignment. Answers Students may give more comprehensive answers that the minimum listed below and they may include photographs of simple AAS and AES spectra. 1. Source a commercially available GFAA. Student must choose one commercially available GFAA. 2. Give full details of make, model and a photograph of the equipment. Student should provide enough details for the tutor to find additional materials pertinent to this GFAA if required. 3. List and describe the important features of this GFAA according to the manufacturer’s information. A list of the selling features is sufficient but the student needs to describe these features rather than just list them. 4. Obtain a schematic diagram of the components of this GFAA or a similar GFAA. Label the major components on this diagram and describe briefly what each component does. Note: at this stage you have not covered all components of AAS equipment in detail. You should consider this question as a preview of what is to come in further sections of this section. Should be essentially the same as that contained in Study Notes: Graphite Furnace Atomiser. The student needs to describe what each major component does. 5. Find and detail one application for this GFAA. An application is essentially an SOP developed by the manufacturer describing how to use their equipment to measure a specified analyte. Student needs to supply a copy of this for the information of the Tutor. 6. What are the important/crucial steps in this application and why? Do not just list them all. Student is required to identify the important steps in the application and why they consider them to be essential. There may be a wide variation in answers here dependent on the knowledge and application of the student to the task. 7. Give three reasons why GFAA is better than flame AAS? Straight form the Table in Study Notes: Graphite Furnace Atomiser. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 27 Laboratory Operations Toolbox Teacher Guide Section: 4. Task: 2. Step: 4. Activity: Assignment – AAS Monochromator Questions You will need to participate in a discussion forum in order to answer the questions below. Go to the Discussion Forum: AAS Monochromator to discuss this AAS topic with specific relevance to the questions posed below: 1. What is a monochromator and how does it work? 2. What are the common problems associated with the use of a monochromator? 3. How are these problems overcome? 4. What is the relationship between slit width, light throughput, signal-to-noise ratio and resonance line isolation? You are expected to contribute at least three times to the forum. Use the button marked Forum on the main page. Send your answers to your tutor along with a printout of the discussion forum in which you have participated at least three times. Answers Students may give a range of answers from brief to very descriptive based on their experience with AAS, their work ethic and the composition of their particular discussion forum. 1. What is a monochromator and how does it work? A monochromator produces monochromatic light via use of a diffraction grating and is used in AAS to produce incident light of very specific wavelength. It works via a diffraction grating that diffracts light selectively. 2. What are the common problems associated with the use of a monochromator? Incorrect selection of slit width Incorrect selection of wavelength Resonance lines very close in wavelength for particular elements. 3. How are these problems overcome? Select correct width – may be a compromise between resonance and light throughput and hence signal-to-noise ratio. Select correct wavelength Choose another line if possible of use a monochromator with a better bandpass (0.02 nm is typical but 0.01 nm or better are available), respectively 4. What is the relationship between slit width, light throughput, signal-to-noise ratio and resonance line isolation? Students should be able to clearly outline this based on the Study Notes. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 28 Laboratory Operations Toolbox Teacher Guide Section: 4. Task: 2. Step: 6. Activity: Assignment – Types of Spectrophotometer Questions The questions that follow are based on information available on the internet. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. 1. Source one example of a single beam and one example of a dual beam AAS. Both spectrophotometers should be, or have been, commercially available. 2. Give full details of make, model and a photograph of each. 3. List and describe the important features of each relating to the optical system, i.e. relating specifically to the single or dual beams. 4. Obtain a schematic diagram of the beam paths of each. Label the major components on each diagram and describe briefly what each of these components does. 5. What are the basic differences between using each AAS? 6. Are there any specific maintenance or troubleshooting aspects specific to either piece of equipment? Send your answers to your tutor along with a copy of any materials that you have used for this assignment. Answers Learners may give more comprehensive answers that the minimum listed below. 1. Source one example of a single beam and one example of a dual beam AAS. Both spectrophotometers should be, or have been, commercially available. Students should give an example of each even if it based on text book diagrams. They may have difficulty in finding information on single beam AAS. 2. Give full details of make, model and a photograph of each. Additional information for the Tutor. 3. List and describe the important features of each relating to the optical system, i.e. relating specifically to the single or dual beams. Students should be able to explain the basic differences between the two systems. 4. Obtain a schematic diagram of the beam paths of each. Label the major components on each diagram and describe briefly what each of these components does. Student should be able to correctly label and describe major components. There may be some variation in interpretation of ‘major’ but you should expect diagrams that contain the main features described in the Study Notes: AAS Single Beam Design and Study Notes: AAS Dual Beam Design. 5. What are the basic differences between using each AAS? Teacher Guide PMLTEST506 Apply Spectrometric Techniques 29 Laboratory Operations Toolbox Teacher Guide Answer that specifically emphasises the ease of blanking in Dual and more complex blanking in Single. 6. Are there any specific maintenance or troubleshooting aspects specific to either piece of equipment? May be anything specific to the equipment chosen but if not mentioned above, the student should outline the drift problems associated with Single Beam units. Section: 4. Task: 3. Step: 1. Activity: Assignment – Analytical Wavelength and Solvents Questions The questions that follow are based on information available on the internet. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. Describe one practical example of each of the following techniques to increase effective working range of analyses (i.e. decrease sensitivity of detection) when using flame AAS: use of less sensitive wavelength, and Burner Rotation. Describe one practical example of the following technique to decrease effective working range of analyses (i.e. increase sensitivity of detection) when using flame AAS: use of chemical solvents. Describe one practical example of the following technique to decrease effective working range of analyses (i.e. increase sensitivity of detection) when GFAA: use of sequential additions of sample. For each example be sure to include: 1. a copy of the application outlining the technique 2. the analyte under investigation the sample type and sample matrix (if appropriate) 3. your explanation of how and why the technique works 4. details of increase/decrease of sensitivity obtained in terms of relative sensitivity or working range decrease or increase. Send your answers to your tutor along with a copy of any materials that you have used for this assignment. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 30 Laboratory Operations Toolbox Teacher Guide Answers Students should include an example of each of the four listed techniques along with all details asked for. There will be great variation in the answers obtained depending on the source of the information used by the student. Look for an answer that is clear, concise and that demonstrates an understanding of the basics of the topic. Students should be clear that a decrease in sensitivity leads to an increase in working range and vice versa. Understanding exceeding the materials and theory contained in Study Notes: Analytical Wavelength and Solvents is not required. Section: 4. Task: 3. Step: 2. Activity: Assignment – AAS Deviations from Beer’s Law Questions The questions that follow are based on information that was not contained in the Study Notes: Deviations from Beer’s Law. You will need to search the Internet, consult appropriate textbooks or other sources to answer the questions. 1. Find information on the Standard-Addition Method that is used to apply a correction factor to results when an assayed standard is found to deviate from the original calibration value of the standard. 2. Explain how the Standard-Addition Method works. 3. In your answer be sure to include: a. a description of why the method is used b. a description of how the method is used, but c. there is no need to include complex mathematical expressions in your answer. Send your answers to your tutor along with a copy of any materials that you have used for this assignment. Answers There may be variation in the answers obtained depending on the source of the information used by the student but each answer should contain the points listed below. Look for an answer that is clear, concise and that demonstrates an understanding of the basics of the topic. The use of standard additions involves preparation of standards from the actual samples. i.e. using the sample matrix to prepare the standards. This minimises the Teacher Guide PMLTEST506 Apply Spectrometric Techniques 31 Laboratory Operations Toolbox Teacher Guide mismatch of physical and chemical parameters between the standards and the samples. The method assumes that the added analyte will be affected by interference in the same way as the analyte in the sample. This holds true for physical interferences but there may be other methods used in the case of chemical interferences such as the use of a hotter flame or a releasing agent. In essence the procedure involves: 1. Taking several aliquots of the sample 2. Typically the standards should contain 0% (sample with no addition), 50%, 100% and 150% of the expected concentration of the analyte of interest. 3. The resultant absorbances are plotted against the added concentrations to form a Standard-Addition Calibration. 4. Note that the calibration curve must be extrapolated back to zero as standard 0% will contain the analyte of interest. Sometimes this can be done on the computer interface of the AAS and the instrument will automatically provide correction to sample results as they are measured. 5. Addition Standards are still required to be substantially linear to allow accurate regression to zero. 6. It is also essential to establish an accurate baseline using an appropriate reagent blank. 7. Commonly, each sample must be analysed individually against a set of addition standards that are specific for that sample. 8. When all samples in a batch are chemically and physically similar, the one addition calibration can be used for all. Section: 4. Task: 4. Step 1. Activity: Assignment – Analysing on the Virtual Flame AAS- Part 1 Questions This activity is based on the simulated analysis of water run off for Cu using the virtual flame spectrophotometer. Answer the questions posed below. Cu Standard Concentration 1. Prepare a calibration curve for the Cu standards (draw to scale and label fully). Concentration is on the X-axis and absorbance is on the Y-axis. 2. What is the concentration of Cu in the four diluted samples? Express the result in ppm and remember to take into consideration the dilution factor of the sample. The result should be expressed as ppm of the undiluted sample. Record your answers in the following Table: Teacher Guide PMLTEST506 Apply Spectrometric Techniques 32 Laboratory Operations Toolbox Teacher Guide Solution Standard 1 Standard 2 Standard 3 Standard 4 SL2003/755 SL2003/755 SL2003/755 SL2003/755 Dilution of Sample ----------------Undiluted 75% 50% 25% Absorbance Cu Concentration ppm Note: The absorbance of one or more of the diluted samples may fall outside the range of the Calibration curve. Do not attempt to calculate the result. Rather list the result as not determined. All analytical procedures have inherent variability and as such you should not expect that the results for individual diluted samples will be exactly the same. Answers: Results should look like: Solution Standard 1 Standard 2 Standard 3 Standard 4 SL2003/755 SL2003/755 SL2003/755 SL2003/755 Dilution of Sample ----------------Undiluted 75% 50% 25% Absorbance 0.109 0.201 0.417 0.794 1.155 0.863 0.573 0.281 Cu Concentration ppm 150 300 600 1200 Not determined Not determined ~860 ppm ~420 ppm Thus we have two readings of the Cu concentration ~830 ppm at a 50% dilution and ~420 ppm at a 25% dilution. The readings for the undiluted sample would be: 830 ppm X 2 (to account for dilution factor) = 1660 ppm, and 420 ppm X 4 (to account for dilution factor) = 1680 ppm. These two figures are close enough to consider the analytical run a success. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 33 Laboratory Operations Toolbox Teacher Guide Section: 4. Task: 4. Step: 1. Activity: Assignment – Analysing on the Virtual Flame AAS – Part 2 Questions This activity is based on the virtual flame AAS equipment and your knowledge of flame AAS gained though study of this section. 1. When performing analysis for copper in waste water run off what element should the cathode be constructed of in the hollow cathode lamp? 2. In general terms what would happen to the absorbance readings for a flame AAS that has been set up for optimal performance if: a. the cathode element was changed b. the monochromator slit was changed c. the optical path of the incident beam was changed with respect to the flame d. the flame burner was moved so that the flame position was changed with respect to the optical path e. there were spectral interferences in the sample. how would you rectify this? f. there were chemical interferences in the sample. how would you rectify this? g. you changed to an nitrous oxide-acetylene flame? 3. You wish to clean the burner in the flame AAS after running the spectrophotometer for two hours. What safety precautions should you take before doing this? 4. You suspect that there is something blocking the optical path from the monochromator to the flame or from the flame to the detector. What safety precautions would you take before doing this? 5. You detect the smell of a sickly sweet gas during flame AAS analysis of water for Cu. What is the most likely source of this smell and what would you do to investigate and rectify the situation? Answers 1. When performing analysis for copper in waste water run off what element should the cathode be constructed of in the hollow cathode lamp? Cathode would be made of copper. Some hollow cathode tubes have multiple elements and in such a case one containing Cu would be required. 2. In general terms what would happen to the absorbance readings for a flame AAS that has been set up for optimal performance if: a. The cathode element was changed – Absorbance would change b. The monochromator slit was changed – Absorbance would change c. The optical path of the incident beam was changed with respect to the flame – Absorbance would decrease d. The flame burner was moved so that the flame position was changed with respect to the optical path – Absorbance would decrease Teacher Guide PMLTEST506 Apply Spectrometric Techniques 34 Laboratory Operations Toolbox Teacher Guide e. There were spectral interferences in the sample. How would you rectify this? Absorbance would decrease, if appropriate target a different resonance line, use a blank and correct readings for the blank, vary temperature or fuel-to-oxidant ratio, or use a radiation buffer. f. There were chemical interferences in the sample. How would you rectify this? Absorbance may increase or decrease. Use a releasing agent, use a protective agent, or use a ionisation suppressor. g. You changed to an nitrous oxide-acetylene flame? Not appropriate for analysis of Cu – absorbance will change in an unpredictable way. 3. You wish to clean the burner in the flame AAS after running the spectrophotometer for two hours. What safety precautions should you take before doing this? Turn off the flame and allow to cool if possible. Don heat protective gloves and be very careful around the atomiser assembly. 4. You suspect that there is something blocking the optical path from the monochromator to the flame or from the flame to the detector. What safety precautions would you take before doing this? Initially check visually but wear UV protective glasses. Turn off flame to investigate further. Wear heat protective gloves and take care around the atomiser assembly. 5. You detect the smell of a sickly sweet gas during flame AAS analysis of water for Cu. What is the most likely source of this smell and what would you do to investigate and rectify the situation? This is most likely to be acetylene. Turn off the flame and check all connections from the acetylene gas cylinder to the internal pipework for leaks. Check all connections to the AAS and check all internal connections inside the AAS cabinet. If no leaks found turn the flame back on and check flame stoichiometry is correct for this application and check acetylene pressure. Section: 4. Task: 5. Step: 1. Activity: Assignment – Reporting Results Questions Based on the requirements in Test Result Reporting Procedure (Quality Manual) prepare a suitable proforma for a Laboratory Test Sheet that might be used by SimuLab. Show all required sample details/test results and customer details, fill the form for the analysis of the water run off for Cu. Make up any details that are not provided. Be sure to design a form that includes raw and final results and that details calculations. Send the completed report to your tutor Answer Tutor to provide feedback based on proforma produced by learner. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 35 Laboratory Operations Toolbox Teacher Guide Section: 5. Task: 4. Step: 1. Activity: Assignment – Interpretation of Spectra Question There are two problems to work through in this assignment: In each of these examples there is a Mass Spectrum, IR Spectrum and an NMR Spectrum. (refer to online version for graphs) Use your knowledge of the techniques of MS, IR and NMR to determine the structure and identity of each of these four organic compounds. Your answer for each compound should include: Your interpretation of the information from each of the three spectra What this information means in regards to the composition and structure of each compound How this information is used to identify the compound Your answer – name and structure of compound. Send your answers to your tutor. Answer Problem No. 1 Mr = 86.1366 Mr = 86.1366 means that the molecular formula is C5H10O The molecular formula rules out the possibility of amine (no N present), ester (requires two Oxygen) and carboxylic acid (requires two Oxygen). IR strong absorption at 2900 is probably Nujol 1740 is probably aldehyde RCHO or ketone RCOR’ but not ester or carboxylic acid as these are already ruled out. The compound is most likely an aldehyde but could possibly be a ketone even though the absorption is at a slightly higher wavenumber than normal. Possible aldehyde isomers: CH3CH2CH2CH2CHO (CH3)2CHCH2CHO (CH3)3CHCHO CH3CH2CH(CH3)CHO Possible ketone isomers: CH3CH2COCH2CH3 CH3CH2CH2COCH3 (CH3)2CHCOCH3 NMR Triplet at 2.5 integrates as 2 Singlet at 2.2 integrates as 3 Multiplet at 1.3 integrates as 2 Triplet at 0.8 integrates as 3 As there is no peak integrating as 1 the structure is not an aldehyde which would have – CHO. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 36 Laboratory Operations Toolbox Teacher Guide There are three equivalent protons at 2.2 suggesting a CH3 group. As this is a singlet there is no adjacent C with an attached H. This is consistent with a ketone carbonyl group attached to this CH3 such as C – CO – CH3 which is further supported by the chemical shift of 2.2 There are three equivalent protons at 0.8 suggesting a CH3 group. As this is a triplet the neighbouring carbon must have two protons such as CH2 This is consistent with a CH3 - CH2 - structure. The two equivalent protons at 2.5 split as a triplet indicates CH2 with the neighbouring carbon having two protons. This is consistent with - CH2 - CH2 –CO The multiplet at 1.3 integrating as two equivalent protons is indicative of a CH2 group with multiply protonated carbons on both sides. This is consistent with CH3 - CH2 - CH2 Hence the most likely structure is 2-prpoanone: CH3 - CH2 - CH2 - CO - CH3 The mass spectrum shows: 71 M-15 for cleavage of terminal CH3 43 for both CH3 - CH2 - CH2 - and - CO - CH3 fragments This is entirely consistent with the structure of 2-prpoanone. Answer Problem No. 2 Mr = 62.0696 Mr = 62.0696 means that the molecular formula is C2H6O2 The molecular formula rules out the possibility of amine (no N present). The presence of two oxygens allows for the possibility of carboxylic acid, ester or multiple alcohol, aldehyde, ketone or ether groups. IR strong absorption at: 1430 suggests an – O- H band in carboxylic acid However, there is no carbonyl peak so it is unlikely to be carboxylic acid. The peak is near to that expected for - C – O absorption found in alcohol or ether but not ester since there is no carbonyl absorption. The compound is most likely a diol or diether. Possible alcohol isomers could include: CH3CH(OH)2 HOCH2CH2OH Possible ether isomers could include: CH3- O – OCH3 However, this is a peroxide and not an ether at all so it may be ruled out. NMR Ther are two peaks that integrate in the ratio 1:2 As there are six hydrogens in the molecular formula, these peaks must correspond to 2 and 4 equivalent protons respectively. Singlet at 4.6 integrates as 2 Teacher Guide PMLTEST506 Apply Spectrometric Techniques 37 Laboratory Operations Toolbox Teacher Guide Singlet at 3.8 integrates as 4 The possible structure CH3CH(OH)2 would produce three NMR peaks in the ratio 1:2:3 representing the protons associated with CH, (OH)2 and CH3 respectively so this structure can be ruled out. There are four equivalent protons at 3.8 suggesting two CH2 groups. As this is a singlet the two CH2 groups experience identical environments where neighbouring atoms could be O bonded to H. This is consistent with HOCH2CH2OH This structure is consistent with the peak at 4.6 for two – O – H groups. The mass spectrum shows: 31 M-31 for cleavage of HO – CH2 - fragment 61 M-1 for removal of one terminal H from – O - H group 60 M-2 for removal of two terminal Hs from – O - H 44 M-18 for removal of – O – after terminal Hs have been removed. The molecular formula is HOCH2CH2OH 1,2-ethane diol (ethylene-1,2-diol) Teacher Guide PMLTEST506 Apply Spectrometric Techniques 38 Laboratory Operations Toolbox Teacher Guide SECTION 6 – Final Assessment Part 1. Knowledge Assessment Final Assessment Section 1 – Prepare for the Analytical Procedures Questions and Answers 1. How would you confirm the identity of a sample for analysis and how would you locate the standard method to be employed? By use of a unique laboratory number attached to the sample, sample documentation, appearance and form of the sample and seek further assistance from other laboratory staff or the customer if unsure. Determine standard method by consulting Methods manual or appropriate SOP, ask assistance from other laboratory staff or use method as supplied by the customer. 2. Name three hazards associated with the use of gas cylinders in the laboratory. Heavy and awkward, high pressure, propelled if uncontrolled release of gas, flammable, explosive, corrosive, poison, asphyxiating and cold burns. 3. Sample condition is important for analysis. Name three potential problems arising from sample condition. Chemical contamination, microbial contamination, stored at incorrect temperature, stored under adverse conditions e.g. light, heat or dust, leaking, deterioration, colour change, evaporation and absorption of moisture. 4. Why is chain of custody of samples important? Chain of custody is often used for samples when the results may be used in a court of law or other legal proceedings. It is a system that monitors the flow and integrity of samples. For example analysis of possible drugs of dependence, explosives, fuels or commercial material where substitution is suspected. 5. Sample preparation is also important for the subsequent analysis to be accurate. Use an example from your own experience and explain and detail each of the steps involved in the sample preparation. Be sure to explain why each of these steps is important and how incorrect preparation may affect the sample and the subsequent analysis. The answer given will depend on the sample preparation method chosen but you should look for an answer that is clear, concise and demonstrates an understanding of the process and potential impacts on the integrity of the sample and accuracy of analytical results. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 39 Laboratory Operations Toolbox Teacher Guide Final Assessment Section 2 – Spectroscopic Concepts and Principles Questions and Answers 1. Give a brief explanation of spectroscopy. Spectroscopy is about the light an object absorbs or emits and its relationship to the composition of the object. 2. In spectroscopy light is absorbed or emitted by what? By the atoms and molecules of the substance. 3. What is electromagnetic radiation (EMR) and what is it comprised of? EMR is a form of energy and comprises X-rays, UV light, visible light, infrared, microwaves, and television/radio waves. 4. What is Beer’s Law and why is it important? Beer’s Law is given as A = Elc where A = absorbance, c = concentration, l = optical path length and E the molar absorptivity constant. It is important because for various ranges of concentration of a substance, the relationship between absorbance and concentration is linear. This allows the use of standard solutions and the construction of a standard curve to calculate the concentration of an unknown solution. 5. How are transmittance (T) and absorbance (A) related? They are inversely related as demonstrated by the following equations: A = - log T and T = 10-A. 6. If A = 0.3, what does T equal, and when T = 25%, what does A equal? T = 10-0.3 = 50%; A = -log 0.25 = 0.6 7. A sample solution with a molar absorbency of 210 cm-1 mol –1 L gives an absorbance reading of 0.612 in a 1.0cm curette. What is the molar concentration of the anolyte? C = A/EL = 0.0029M Final Assessment Section 3 – UV-Vis Spectroscopy Questions and Answers 1. What range of wavelength is UV and Visible light? UV = 200 -380 nm, Visible = 380 -780 nm. 2. How does an anolyte absorb and emit energy in UV-Vis spectroscopy? A chemical species absorbing a quantum (packet) of light energy leading to an electron moving to a higher energy orbital and the reverse of this process where the electron drops back to a lower energy level and the chemical species emits a miniscule amount of heat. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 40 Laboratory Operations Toolbox Teacher Guide 3. What parameters affect UV-Vis absorption by an organic compound? The sample solvent, the concentration of the sample, the pH of the sample and the temperature of the sample. 4. In UV-Vis spectrophotometry what is the sample held in and what care needs to be taken to ensure correct readings? The sample is held in a cuvette or cell. There are a number of factors that may reduce the accuracy of the measurement. These include: leaving the sample out, not filling the cuvette enough, not aligning the cuvette correctly, fingerprints on the cuvette, dirty cuvette, scratched cuvette, not closing the cuvette holder lid, evaporation from volatile samples, sample that is corrosive to the cuvette material, using single use cuvettes more than once and air bubbles in the cuvette. 5. UV-Vis spectrophotometers come as single beam or dual beam designs. What is the major advantage of a dual beam design? Allows for automatic blanking where the blank is left in the spectrophotometer and only the sample is changed for each reading. 6. Why is choice of solvent important in UV-Vis spectrophotometry and what wavelength would be the minimum that you could use for the following two sample solvents – acetone and diethyl ether? The choice of a sample solvent for UV-Vis spectroscopy is important because certain solvents adsorb light below a minimum wavelength. If the analytical wavelength required for the sample is below this minimum wavelength then inaccurate readings of the sample will occur. Acetone – 330 nm, diethyl ether – 210 nm. 7. Beer’s Law describes the relationship between concentration and absorbance. What causes deviations from Beer’s Law? Only works with dilute solutions, at higher concentrations there are interactions between absorbing particles and the refractive index of the solution may alter causing problems with the linearity of Beer’s Law. Instrumental factors may also be involved and these include: stray light, electronic noise, polychromatic radiation, incorrect selection of analytical wavelength and at very low concentrations the instrument may be inaccurate. Final Assessment Section 4 – Atomic Absorption Spectroscopy: A Scenario Questions and Answers You have been asked to determine the Pb level of the water run off sample that was used in section 4 for Cu analysis. 1. Explain how you would do this in general terms, i.e. explain the general method of flame AAS. Expect an answer that outlines the general concepts of flame AAS. The student should be aware that changing from Cu to Pb analysis is not very difficult and only involves the changes listed in the next question. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 41 Laboratory Operations Toolbox Teacher Guide 2. Specify and list those flame AAS parameters that would need to be changed to perform this analysis. Hollow Cathode Lamp with a Pb cathode Wavelength of 217.0 nm New Pb standards required Flame remains the same type Adjust burner and monochromator position as required. 3. List any additional safety requirements for the analysis of lead over those required for the analysis of Cu. Trick question – there are no additional safety requirements. Samples containing copper should be treated in the same safe manner to those containing Pb although Pb is the more toxic metal. Student may mention production of explosive copper acetylides but this would not happen from the sample. 4. After analysis you find that the concentration of Pb in the water run off is very low and you suspect that your measurements at this concentration are inaccurate. List two ways that you could use to overcome this problem. Note – one of the ways should be a change of equipment. Concentrate the sample by evaporation or other means. Use graphite furnace AAS using sequential addition and ashing prior to atomisation. The learner will need to search the Internet, consult appropriate textbooks or other sources to answer the following questions. 5. What flame type would you use for Pb analysis? Acetylene-Air, same as for Cu. 6. In µg/mL, what are the working ranges for Cu and Pb for flame AAS analysis? Questions 6 and 7 may take the student some effort to find but should not be unduly difficult. There may be some minor variations in the numbers quoted depending on the source of their information. Cu: 0.02 – 10 µg/mL Pb: 0.2 – 30 µg/mL 7. In ng/mL, what are the working ranges for Cu and Pb graphite furnace AAS analysis? Cu: 1 – 20 ng/mL Pb: 2 – 25 ng/mL 8. What are the advantages of the graphite furnace AAS over the flame AAS? Greater sensitivity as evidenced above Teacher Guide PMLTEST506 Apply Spectrometric Techniques 42 Laboratory Operations Toolbox Teacher Guide Able to add sample sequentially prior to atomisation High conversion efficiency of sample into free ions Absorption signal is a well defined peak Peak heights and areas can be used for quantitative analysis Temperature is only factor involved in free atom production Allows analysis of liquid samples that would be inappropriate for flame AAS Low spectral interference due to generally higher temperatures. 9. What general laboratory safety precautions would you take when working with water run off containing unknown concentrations of Cu and Pb, as well as other possible contaminants. Standard PPE of laboratory coat, covered footwear, safety glasses and possibly latex gloves UV Safety Glasses and heat resistant gloves as appropriate when setting up the AAS and when running samples Avoid contact of sample with skin or inhaling vapours during acidification of samples or atomisation of samples Require proper ventilation If appropriate, take precautions against microbiological hazards associated with water run off, e.g. sewage contaminated water. Final Assessment Section 5 – Structural Elucidation Questions and Answers 1. What is structural elucidation? Structural elucidation is the determination of the composition and structure of an organic molecule using spectra derived from analysis of the compound using Mass Spectroscopy, Infrared Spectrophotometry and Nuclear Magnetic Resonance Spectrometry. 2. Briefly explain how a mass spectrometer (MS) works. Sample molecules are introduced into an ionisation chamber where a beam of electrons ionises the sample molecules to mainly positively charged ions. These ions are accelerated in an electric field then passed through a magnetic field to curve their flight path. The radius of curvature depends on the mass/charge ratio of the ions and the magnetic field is changed to allow detection of different streams of charged ions. This produces a mass spectrum that gives information about the composition and structure of the compound. 3. What would be the detected masses of the following molecules in a mass spectrometer – C2H8N2, CH4, C4H8O3? C2H8N2 – 60.1002, CH4 – 16.0434, C4H8O3 = 104.1080. 4. Briefly explain how an infrared spectrophotometer (IR) works? Teacher Guide PMLTEST506 Apply Spectrometric Techniques 43 Laboratory Operations Toolbox Teacher Guide IR uses similar instrumentation to UV-Vis spectrophotometry except it uses a different light source comprising infrared radiation at frequencies of ~1011 to 1014 Hz. It uses a split beam – reference and analytical beam. The analytical beam passes through the sample so that molecular vibrational absorption of the infrared energy occurs. The two beams are compared and the result is an IR spectrum consisting of downward facing peaks, i.e. IR measures absorption of infrared radiation. 5. Briefly explain how a nuclear magnetic resonance spectrometer (NMR) works? The NMR detects the nuclear spin of molecular species via the use of radio frequency (RF) to ‘flip the spin’ of the nuclei in the spinning sample container in a strong magnetic field. 6. a) Interpret the following NMR spectrum for ethanol by relating the features of the spectrum to the structure of the molecule. Mr = 46.0702 (refer to online version for graph) The final structure is already known to be ethanol: CH3CH2OH. NMR Singlet at 4.0 integrates as 1 Quadruplet at 3.5 integrates as 2 Triplet at 1.2 integrates as 3 There are three equivalent protons at 1.2 suggesting one CH3 group. The chemical shift agrees with CH3-C-O Since the absorption is a triplet, there is most likely an adjacent CH2 group. This is consistent with the CH3CH2OH structure. There are two equivalent protons at 3.5 The quadruplet split indicates an adjacent CH3 group This is consistent with the CH3CH2OH structure. The single proton at 4.0 is consistent with –OH. The singlet split indicates the proton is not attached to carbon as in CH3CH2OH b) Interpret the following MS and IR data for enthonol by explaining how the information is related to the structure (or formula) of the molecule. (refer to online version for graphs) The final structure is already known to be ethanol: CH3CH2OH. IR strong absorption at 3000 is probably Nujol 3620 is probably alcohol O-H 1080 is probably alcohol C-O Hence, the IR data is consistent with ethanol. The mass spectrum shows: 45 M-1 for removal of H to leave possible CH3CH2O fragment 31 M-15 for removal of CH3 to leave possible CH2OH fragment Teacher Guide PMLTEST506 Apply Spectrometric Techniques 44 Laboratory Operations Toolbox Teacher Guide 7. Determine the structure of the molecule based on the following MS, IR and NMR data. (refer to online version for graphs) Mr = 88.1086 means that the molecular formula is C4H8O2 The molecular formula rules out the possibility of amine (no N present). The presence of two oxygens allows for the possibility of carboxylic acid, ester or multiple alcohol, aldehyde, ketone or ether groups. IR strong absorption at: 1710 is possible carbonyl group of aldehyde, ester or carboxylic acid 1380 could belong to – C – O – of ester or - O – H of carboxylic acid. There is no peak in the vicinity of 2830 – 2695 for aldehyde - C - H The compound is most likely an ester or carboxylic acid. Possible ester isomers could include: CH3CH2COOCH3 CH3COOCH2CH3 Possible carboxylic acid isomers could include: CH3CH2CH2COOH (CH3)2CHCOOH NMR Quadruplet at 4.2 integrates as 2 Triplet at 2.0 integrates as 3 Singlet at 1.2 integrates as 3 As there is no peak integrating as 1 the structure is not a carboxylic acid which would have – COOH. Hence, ester is the most likely compound. There are three equivalent protons at 2.0 suggesting a CH3 group. As this is a singlet the neighbouring atom (C or O) is not bonded to H. This is consistent with an ester configuration such as C – CH3 There are three equivalent protons at 1.2 suggesting a CH3 group. As this is a triplet the neighbouring carbon must have two protons such as CH2 This is consistent with a CH3 - CH2 - structure. The two equivalent protons at 2.5 split as a triplet indicates CH2 with the neighbouring carbon having two protons. This is consistent with - CH2 - CH2 – CO There are two equivalent protons at 4.2 suggesting a CH2 group. As this is a quadruplet split there must be three neighbouring protons such as a CH3 group. This is consistent with CH3 - CH2 - C – or CH3 - CH2 - O Possible structures are CH3 - CH2 - COO - CH3 or CH3 – COO - CH2 - CH3 The mass spectrum shows: 29 for the CH3 - CH2 - fragment 73 M-15 for removal of CH3 fragment 59 M-27 for removal of CH3CH2 fragment 43 M-45 for cleavage of CH3CH2 – O fragment As there is no cleavage of – OCH3 but there is a strong peak for removal of – OCH2CH3 the structure is most probably ethyl acetate: CH3COOCH2CH3 Teacher Guide PMLTEST506 Apply Spectrometric Techniques 45 Laboratory Operations Toolbox Teacher Guide Part 2. Practical Assessment Practical assessment requires you to demonstrate the competency on-the-job (or in a simulated laboratory). The assessment will consist of: a demonstration of the competency in an on-the-job situation or a simulation oral questioning about such things as laboratory specific knowledge of processes, troubleshooting and questioning related to specific safety or other factors that may be peculiar to the learner's work environment any relevant workplace documents that support the assessment. If you are ready to undertake the practical assessment send a message to your tutor using the mail facility. Note: there are checklists for five (5) analytical techniques on the following pages. Use only those checklists that are appropriate for the analyses that you perform. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 46 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – UV-Vis Spectrophotometry Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using UV-Vis Spectrophotometer Use safety information (e.g. MSDSs and SOPs) to perform Spectrophotometry procedures safely Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration correctly Follows SOP when carrying out analysis Is aware of, and plans for, possible problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment Spectrophotometer is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly N/A Is proactive in operating equipment and in troubleshooting Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations N Sets up spectrophotometer correctly for analysis of a specified analyte Reads SOP Understands SOP Y Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Name of candidate ________________________________ Name of assessor ________________________________ Signature ____________________ Signature ______________________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques Date __________ Date ____________ 47 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – Mass Spectroscopy (MS) Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using MS Use safety information (e.g. MSDSs and SOPs) to perform MS procedures safely Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration if required Follows SOP when carrying out analysis Is aware of, and plans for, possible problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment MS equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly N/A Is proactive in operating equipment and in troubleshooting Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations N Sets up MS equipment correctly for analysis of a specified analyte Reads SOP Understands SOP Y Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Name of candidate ________________________________ Name of assessor ___________________________________ Signature ____________________ Signature ______________________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques Date __________ Date ____________ 48 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – Infrared Spectrometry (IR) Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using IR Use safety information (e.g. MSDSs and SOPs) to perform IR procedures safely Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration if required Follows SOP when carrying out analysis Is aware of, and plans for, potential problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment IR equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly N/A Is proactive in operating equipment and in troubleshooting Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations N Sets up IR correctly for analysis of a specified analyte Reads SOP Understands SOP Y Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Name of candidate ________________________________ Name of assessor ___________________________________ Signature ____________________ Signature ______________________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques Date __________ Date ____________ 49 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – Nuclear Magnetic Resonance Spectrometry (NMR) Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using NMR Use safety information (e.g. MSDSs and SOPs) to perform NMR procedures safely Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration if required Follows SOP when carrying out analysis Is aware of, and plans for, potential problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment NMR equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly N/A Is proactive in operating equipment and in troubleshooting Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations N Sets up NMR correctly for analysis of a specified analyte Reads SOP Understands SOP Y Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Name of candidate ________________________________ Name of assessor ___________________________________ Signature ____________________ Signature ______________________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques Date __________ Date ____________ 50 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills - Flame AAS Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using flame AAS Use safety information (e.g. MSDSs and SOPs) to perform flame AAS procedures safely Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration correctly Follows SOP when carrying out analysis Is aware of, and plans for, interferences Is able to investigate and rectify interference situations using standard methods Flame AAS equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Clean and maintain equipment N/A Is proactive in operating equipment and in troubleshooting Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations N Sets up flame AAS correctly for analysis of a specified analyte Reads SOP Understands SOP Y Safety Accuracy or legibility. If appropriate to the enterprise, is able to use graphite furnace AAS Understands differences between flame and graphite furnace AAS Can perform GFAA using established methods. YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Name of candidate ________________________________ Name of assessor ___________________________________ Signature ____________________ Signature ______________________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques Date __________ Date ____________ 51 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – UV-Vis Spectrophotometry Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using UV-Vis Spectrophotometer Reads SOP Understands SOP Use safety information (e.g. MSDSs and SOPs) to perform Spectrophotometry procedures safely Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations Sets up spectrophotometer correctly for analysis of a specified analyte Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration correctly Follows SOP when carrying out analysis Is proactive in operating equipment and in troubleshooting Is aware of, and plans for, possible problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment Spectrophotometer is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. Y N N/A YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 52 Laboratory Operations Toolbox Teacher Guide Feedback to candidate: Name of candidate ________________________________ Name of assessor ______________________________ ____________ Signature Date ____________________ Signature __________ Date ______________________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques 53 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – Mass Spectroscopy (MS) Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using MS Reads SOP Understands SOP Use safety information (e.g. MSDSs and SOPs) to perform MS procedures safely Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations Sets up MS equipment correctly for analysis of a specified analyte Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration if required Follows SOP when carrying out analysis Is proactive in operating equipment and in troubleshooting Is aware of, and plans for, possible problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment MS equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. Y N N/A YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Teacher Guide PMLTEST506 Apply Spectrometric Techniques 54 Laboratory Operations Toolbox Teacher Guide Name of candidate ________________________________ Name of assessor Signature Date ____________________ Signature ___________________________________ ____________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques __________ Date ______________________ 55 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – Infrared Spectrometry (IR) Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using IR Reads SOP Understands SOP Use safety information (e.g. MSDSs and SOPs) to perform IR procedures safely Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations Sets up IR correctly for analysis of a specified analyte Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration if required Follows SOP when carrying out analysis Is proactive in operating equipment and in troubleshooting Is aware of, and plans for, potential problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment IR equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. Y N N/A YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Teacher Guide PMLTEST506 Apply Spectrometric Techniques 56 Laboratory Operations Toolbox Teacher Guide Name of candidate ________________________________ Name of assessor Signature Date ____________________ Signature ___________________________________ ____________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques __________ Date ______________________ 57 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills – Nuclear Magnetic Resonance Spectrometry (NMR) Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using NMR Reads SOP Understands SOP Use safety information (e.g. MSDSs and SOPs) to perform NMR procedures safely Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations Sets up NMR correctly for analysis of a specified analyte Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration if required Follows SOP when carrying out analysis Is proactive in operating equipment and in troubleshooting Is aware of, and plans for, potential problems Is able to investigate and rectify problem situations using standard methods Clean and maintain equipment NMR equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. Y N N/A YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Teacher Guide PMLTEST506 Apply Spectrometric Techniques 58 Laboratory Operations Toolbox Teacher Guide Name of candidate ________________________________ Name of assessor Signature Date ____________________ Signature ___________________________________ ____________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques __________ Date ______________________ 59 Laboratory Operations Toolbox Teacher Guide Collection of Evidence Observation checklist for demonstration of skills - Flame AAS Name of Candidate: …………………………………………………………… During the demonstration of skills, did the candidate: Apply SOPs to efficiently prepare samples for test and analyses using flame AAS Reads SOP Understands SOP Use safety information (e.g. MSDSs and SOPs) to perform flame AAS procedures safely Clothing and safety equipment are maintained to meet work area standards Understands and applies safety principles Can troubleshoot hazardous or potentially hazardous situations Sets up flame AAS correctly for analysis of a specified analyte Test equipment is set up according to established methods Uses appropriate standards and sets up Standard Calibration correctly Follows SOP when carrying out analysis Is proactive in operating equipment and in troubleshooting Is aware of, and plans for, interferences Is able to investigate and rectify interference situations using standard methods Clean and maintain equipment Flame AAS equipment is cleaned and maintained according to established methods Follows SOP when carrying out cleaning and maintenance Calculate, record and present results accurately and legibly Records are completed according to enterprise requirements Calculations are correct Complete all tests within required timeline without sacrificing: Safety Accuracy or legibility. If appropriate to the enterprise, is able to use graphite furnace AAS Understands differences between flame and graphite furnace AAS Can perform GFAA using established methods. Y N N/A YES NO The candidate’s overall performance met the standard: NOTE 1: These questions are models ONLY and are NOT comprehensive. You should develop your own panel of questions tailored to your teaching/training situation. Teacher Guide PMLTEST506 Apply Spectrometric Techniques 60 Laboratory Operations Toolbox Teacher Guide NOTE 2: NA means, “Not observed at this time”. Feedback to candidate: Name of candidate ________________________________ Name of assessor Signature Date ____________________ Signature ___________________________________ ____________ Teacher Guide PMLTEST506 Apply Spectrometric Techniques __________ Date ______________________ 61