Vapor Pressure & Enthalpy Lab: Clausius-Clapeyron Equation
advertisement
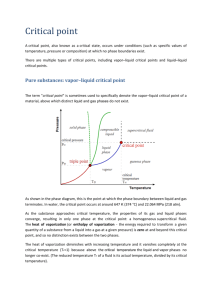
Vapor Pressure and Enthalpy of Vaporization When a liquid is placed in a container, and the container is sealed tightly, a portion of the liquid will evaporate. The newly formed gas molecules exert pressure in the container, while some of the gas condenses back into the liquid state. If the temperature inside the container is held constant, then at some point equilibrium will be reached. At equilibrium, the rate of condensation is equal to the rate of evaporation. The pressure at equilibrium is called vapor pressure, and will remain constant as long as the temperature in the container does not change. This equilibrium relationship between vapor and liquid can be derived from the thermodynamic master equation. (1) dG = VdP – SdT If the liquid and vapor phases are at equilibrium with one another, then ∆G = 0. Rearranging the master equation and substituting in the equation for the change of entropy for vaporization gives a general equation for phase transitions: (2) ∆S = ∆Hvap / T (3) ∫ dP = ∫ ∆H m,Trans T∆Vm dT Equation (3) can be solved by making the assumption that the enthalpy of transition is independent of T & P, and that the change in volume can be attributed solely to the ideal gas volume. With these assumptions in place, the Clausius-Clapeyron equation describes how vapor pressure changes with changes in temperature, as well as describes how the enthalpy of vaporization affects those changes. (4) ln P2 − ∆H vap = P1 R 1 1 − T2 T1 P = vapor pressure, T is Kelvin temperature, ∆Hvap is the molar enthalpy of vaporization, and R is the universal gas constant (in units of J/K*mol). If we let P1 and T1 be fixed and equal to the values for “standard” conditions (Po = 1bar, Tovap = “standard boiling point”), then equation (4) can be re-written for other T & P conditions as − ∆H vap (5) ln P = (6) C = constant = Gentry, 2014 RT +C + ∆H vap o vap RT + ln( P o ) (solving for To, Po=1bar) Theoretical equations are only as good as the assumptions made to derive them. In this case, the three principal assumptions are that: 1) ∆Hvap is constant, independent of both T and P, 2) The volume of the liquid is negligible compared to the volume of the gas, and 3) ∆V is given by the volume of an ideal gas. If the gases do not obey the ideal gas law, then we must correct the Clausius-Clapeyron equation using a “real gas” equation such as compressibility. (7) Z ≡ Vactual / Videal (8) ln P = − ∆H vap Z ⋅ RT or Vactual = Z · nRT/P +C One method we can use to estimate the compressibility factor is the Berthelot equation. (9) 9 PTc Z = 1+ 128 Pc T 2 1 − 6 Tc T where Tc and Pc are the critical temperature and pressure of the liquid taken from the literature. [for example, http://webbook.nist.gov/chemistry] It should be pointed out that the Clausius-Clapeyron equation also assumes that ∆Hvap is independent of temperature. In reality we know that the enthalpy of transition changes as you move away from the standard vaporization temperature. This is due to there being differences in the heat capacities for solids and liquids. (10) T o ∆H vap (T ) = ∆H vap + ∫ o (C p ,vapor − C p ,liquid ) dT T We will be applying the assumption of ∆Hvap=constant over only a small temperature range, so errors due to the assumption should be small. OBJECTIVES In this experiment, you will introduce a specific volume of a volatile liquid into a closed vessel, and measure the pressure in the vessel at several different temperatures. Since there will be residual air in the vessel as well, you will need to use Dalton’s Law of Partial Pressure to extract the vapor pressure from the observed pressure. By analyzing your measurements, you will be able to calculate the relationship between Pvap and T and calculate the ∆Hvap of the liquid. -2- PROCEDURE 1. CAUTION: The alcohol used in this experiment is flammable. Be sure that there are no open flames in the room during the experiment. Notify your teacher immediately if an accident occurs. 2. Connect a Gas Pressure Sensor to Channel 1 of the Vernier computer interface. Connect a Temperature Probe to Channel 2 of the interface. Connect the interface to the computer with the proper cable. Use the clear tubing to connect the white rubber stopper to the Gas Pressure Sensor. (About one-half turn of the fittings will secure the tubing tightly.) 3. Pour 3-5mL of ethanol into an Erlenmeyer flask. Then insert the white stopper snugly into the neck of the Erlenmeyer flask to avoid losing any of the gas that will be produced as the liquid evaporates. Important: Close the valve on the white stopper. 4. Put the stoppered flask in an empty 1 liter beaker that you will later use as a temperature bath. Include a magnetic stir bar so that the liquid can be mixed during the warming process. 5. Start the Logger Pro program on your computer. Open the file “34 Vapor” from the to begin data collection. Advanced Chemistry with Vernier folder. Click 6. When the readings stabilize, click . We will use this room-temperature pressure later to insure that we do not have a gas leak at the top of the flask. 7. AFTER taking a room temperature pressure reading, add ice and water to cover the flask up to the bottom of the stopper. Place the Temperature Probe in the ice bath as well. Monitor the pressure and temperature readings. When the pressure and temperature readings stabilize, click to record the readings. 8. Use the hot plate to start warming your water bath. Do not take any further measurements (other than the initial ice bath pressure) until all of the ice has melted and sufficient mixing has taken place to get uniform temperature across the bath. [You can physically remove most of the ice and replace with cold water to speed the process.] 9. Continue to slowly warm the system, taking temperature/pressure measurements every 35ºC. *** Note the word “slowly”… you want to insure enough time that the temperature of the liquid and vapor inside the flask remains in equilibrium with the temperature of the surrounding water bath. You should have observed a pressure drop when you put the system in ice, and you should have recovered the initial pressure when you got back close to room temperature. If either of these fails to happen, you likely have a pressure leak in your apparatus. 10. Repeat Step 12 until your temperature approaches 40°C. Do not warm the water bath beyond 40°C because the pressure increase may pop the stopper out of the flask. If you must remove some of the water in the bath, do it carefully so as not to disturb the flask. Let me repeat. The stoppers on the flasks are not very secure. The easiest way to have problems is to let gas escape from the stoppers. This will occur if there is too much jostling, especially at higher temperatures where there is excess pressure inside the flask. 11. After you have completed your readings, click to end the data collection. Record the various temperatures and pressures in your lab book. Do not exit Logger Pro until your data are recorded. -3- DATA ANALYSIS 1. Since air was present in the flask before the ethanol was added, the observed pressure inside the flask was a combination of air pressure and ethanol vapor pressure. We therefore need to correct for this air pressure. We know from Dalton’s Law of Partial Pressure that Ptot = Pair + PEtOH. You should be able to browse the internet to find the vapor pressure of ethanol as a function of temperature. Use these literature data to interpolate a value of PEtOH at your lowest temperature. Use Dalton’s Law to extract a value of Pair from your experimental Ptot value at this temperature. You can then use the ideal gas law to adjust Pair for your other temperatures. This will allow you to calculate the resultant vapor pressure (in bar) of just the ethanol as a function of temperature. 2. The Clausius-Clapeyron equation says that a plot of ln(P) vs. (1/T) should have a linear relationship with one another. (a) What are the expected parametric expressions for the slope and the intercept for your data? [I.e., what are the collection of variables that are equal to the slope and intercept?] (b) Using linear regression, obtain your experimental values for slope and intercept and use the slope to calculate your experimental heat of vaporization. 3. Compare your experimental heat of vaporization to a literature value for ethanol 4. Use your graphical Clausius-Clapeyron expression to extrapolate your data to find the “normal boiling point”. [Hint: What are the conditions that define “normal boiling point”?] How does your number compare to the literature value? Comments? 5. The assumption was made that ethanol behaves like an ideal gas. One way to correct for this assumption is to use Eqn. 9 to calculate the compressibility, Z, of your gas. Using Eqn. 9, what is the value of compressibility for ethanol at 298K? If you use this to correct for your calculations, what is your corrected heat of vaporization? (Assume that Z stays constant over the temperature range that we looked at.) 6. Another assumption that was made was that ∆Hvap is independent of temperature. Check this assumption by calculating the value of ∆Hvap at 298K and at 273K? To do these calculations you will need to use the standard heat of vaporization for ethanol. The heat capacities for ethanol are Cp,liq = 112 J/mol*K and Cp,gas = 65.2 J/mol*K? Comment on the accuracy of the ∆Hvap assumption. Reference 1 Advanced Chemistry with Vernier -4-