Don't skip the G phase
advertisement

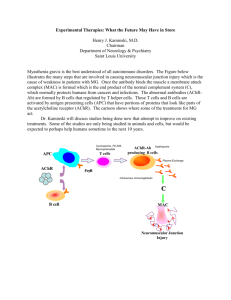
[Cell Cycle 3:7, e27-e29, EPUB Ahead of Print: http://www.landesbioscience.com/journals/cc/abstract.php?id=977; July 2004]; ©2004 Landes Bioscience Don’t skip the G1 phase Extra Views How APC/CCdh1 Keeps SCFSkp2 in Check Cell Cycle 2004; 3(7): http://www.landesbioscience.com/journals/cc/abstract.php?id=977 Once the issue is complete and page numbers have been assigned, the citation will change accordingly. KEY WORDS Skp2, Cks1, p21, p27, APC/C, Cdh1, Cdc20, Emi1, SCF, cell cycle ibu sci ACKNOWLEDGEMENTS No tD ist r This manuscript has been published online, prior to printing for Cell Cycle, Volume 3, Issue 7. Definitive page numbers have not been assigned. The current citation is: The cell cycle is controlled by a family of protein kinases known as cyclin-dependent kinases or CDKs. Timely destruction of CDK subunits and their upstream regulators is an essential means to ensure proper progression through the cell division cycle. Protein degradation is irreversible, and thus constitutes a well suited mechanism to promote the unidirectional passage from one cell cycle phase to the next. In mammalian cells, the transition from G1 to S phase is controlled by the activity of the CDK inhibitors (CKIs) p27 and, to a lesser extent, p21.1 Both CKIs act as negative cell cycle regulators that prevent entry into S phase by inhibiting the activity of Cdk2/Cyclin E, Cdk2/Cyclin A and likely other CDKs. Progression into S phase therefore necessitates the ubiquitin-mediated degradation of the inhibitory proteins. The triggering signal is the CDK-mediated phosphorylation of p27 and p21, allowing the SCFSkp2 (Skp1, Cul1, F box protein Skp2) ubiquitin ligase to specifically recognize the two CKIs as substrates.2,3 SCFSkp2 subsequently catalyzes the polyubiquitination of p27 and p21, thus earmarking both proteins for destruction by the 26S proteasome.3-6 The recognition and efficient ubiquitination of p27 and p21 requires the small accessory protein Cks1 as an indispensable factor in the SCFSkp2 complex.3,7,8 Through its interaction with Skp2, Cks1 may function as an allosteric effector that induces conformational changes in the F box protein to increase its affinity for the substrate, or, more likely, contributes to the binding of phosphorylated p27 by forming a bimolecular interface with Skp2.9,10 As negative regulators of cell proliferation, p27 and p21 intrinsically possess tumor suppressive properties. Hence, the elimination of these impediments to unrestrained proliferation, particularly p27, is a hallmark of many types of cancer.11 Drastically reduced p27 protein levels are frequently found in human malignancies and are often associated with high aggressiveness and poor prognosis. As the antagonist of p27, Skp2 acts as an oncogene, and Skp2 expression was found to inversely correlate with p27 in various carcinomas and lymphomas.11-13 Until recently, the investigation of Cks1 protein levels in cancer has been hampered by the fact that specific antibodies (i.e., antibodies that do not recognize the closely related Cks2 protein) have been unavailable. However, mRNA data and the current limited immunohistochemical data indicate that in some types of cancer overexpression of Cks1 is indeed found, correlating with low levels of p27.14,15 Although more data are needed to evaluate the role of Cks1 in cancer development and progression, it would not be surprising to find that Cks1 has oncogenic properties similar to Skp2 or at least contributes to Skp2 tumorigenicity. This would highlight the need for tight regulation of both Skp2 and Cks1 to avoid unscheduled degradation of p27 and p21. How are Skp2 and Cks1 regulated during an unperturbed cell cycle? It had been previously shown that Skp2 mRNA is present in all phases of the cell cycle, displaying only minor fluctuations.16 Skp2 protein, however, is absent in G0 and G1 cells, accumulates during the G1-S transition and persists through S phase until mitosis. The discrepancy in Skp2 levels between G0/G1 and S phase is due to differences in protein stability; Skp2 protein is very unstable in G0/G1, but upon entry into S phase it is stabilized significantly. However, the mechanism that controls Skp2 stability had not been well understood. Our study and that of Wei et al. have now provided evidence that the degradation of Skp2 in Do Received 05/12/04; Accepted 05/14/04 ce. *Correspondence to: Michele Pagano; Department of Pathology; MSB 599; New York University School of Medicine; New York University Cancer Institute; 550 First Avenue; New York, New York 10016 USA; Tel.: 212.263.5332; Fax: 212.263.5107; Email: michele.pagano@med.nyu.edu By keeping the levels of Skp2 and Cks1 low during G1 progression, APC/CCdh1 prevents unscheduled degradation of SCFSkp2 substrates and premature entry into S phase. Thus, APC/CCdh1, a ubiquitin ligase involved in mitotic exit and maintenance of G0/G1 phase, directly controls SCFSkp2, a ubiquitin ligase involved in the regulation of S phase entry. en New York University School of Medicine; New York University Cancer Institute; New York, New York USA te ABSTRACT Tarig Bashir Michele Pagano* © 20 04 La nd es Bio We thank T. Cardozo for critically reading the manuscript. We apologize to colleagues whose work could not be mentioned due to space limitations. Work in the Pagano lab is supported by grants from the NIH (R01-CA76584 and R01-GM57587). e27 Cell Cycle 2004; Vol. 3 Issue 7 HOW APC/CCDH1 KEEPS SCFSKP2 IN CHECK G0/G1 is mediated by the APC/CCdh1 (anaphasepromoting complex/cyclosome in complex with its activator Cdh1) ubiquitin ligase:17,18 ibu te 1. Overexpression of Cdh1 (but not its homolog Cdc20) in cells leads to a considerable destabilization of Skp2. 2. Depletion of Cdh1 by RNA interference results in the stabilization and accumulation of Skp2 in G0/G1. 3. Cdh1 interacts physically with Skp2. 4. APC/C supplemented with recombinant Cdh1 is capable of ubiquitinating Skp2 in vitro. © 20 04 La nd es Bio sci en ce. Do No tD ist r The APC/CCdh1-mediated degradation of Skp2 is dependent on an N-terminal destruction box (D box) motif (RXXL). Mutation of this motif generates a stable Skp2 protein that is fully active in G1 cells. Intriguingly, Skp2 mutants harboring an inactive D box are still able to interact with Cdh1, suggesting that the D box sequence itself does not function as a landing pad for the Cdh1 moiety of the APC/C. A more detailed analysis of the Skp2 N-terminus revealed that the actual binding site for Cdh1 resides within a stretch of 50 amino acids that is 1. The potential effects of defective Skp2/Cks1 degradation. Normal cell (left panel): located approximately 40 amino acids downstream Figure Cdh1 APC/C is activated in late M/early G1 phase and induces the degradation of the SCF subunits from the D box (amino acids 45–94). In line Skp2 and Cks1. Consequently, the CKIs p27 and p21 accumulate, bind to CDK/cyclin complexes with recent suggestions, the D box may thus and block their activity, thereby ensuring that G1 phase is established and maintained. During the interact with components of the APC/C other G1-S transition, APC/CCdh1 is inactivated through association with Emi1 and CDK-mediated than Cdh1, or it may function as a signalling inhibitory phosphorylation of Cdh1. Skp2 and Cks1 are stabilized, become part of an active sequence that orients the multi-subunit APC/C SCFSkp2 complex and target p27 and p21 for degradation. CDK/cyclin complexes become active and initiate progression into S phase. Cancer cell (right panel): In certain cancer cells APC/CCdh1 complex to the binding site.19 may be unable to interact with Skp2/Cks1 in G1 phase, hence the ubiquitination and subsequent The oscillations and protein stabilities of degradation of both proteins is compromised. As a result, p27 and p21 are targeted for unschedCks1 during the cell cycle parallel those of uled degradation, rendering CDK/cyclin complexes active. The cell progresses faster into S phase Skp2.17 Cks1 was also shown to bind Cdh1, and and DNA replication is prematurely initiated, which can be a cause of genetic instability. overexpression or depletion of Cdh1 in cells have an effect on Cks1 levels similar to Skp2. Although many findings mutant concomitantly stabilized endogenous Cks1 protein. It is thus point to APC/CCdh1 as a direct regulator of Cks1 protein stability, we conceivable that the interaction between Skp2 and Cks1 shields the were unable to ubiquitinate Cks1 in vitro using APC/CCdh1. This latter from ubiquitination and destruction, suggesting that the leaves open the possibility that APC/CCdh1 does not target Cks1 regulation of Skp2 and Cks1 protein stability is closely linked. The directly, but may instead trigger another, APC/CCdh1-dependent, APC/CCdh1-mediated elimination of Skp2 and Cks1 is therefore ubiquitin ligase. Alternatively, an as yet to be identified factor neces- important to maintain the G1 state. In contrast, continuous aberrant sary for Cks1 ubiquitination is missing in the purified preparation of removal of p27 and p21 is likely to be a cause of uncontrolled cell APC/CCdh1. proliferation and may ultimately contribute to the development of Several control mechanisms ensure that the activity of APC/ cancer. The frequently observed overexpression of Skp2 in cancer CCdh1 itself is restricted to late M and G0/G1 phase.20 The complex tissues has in some cases been attributed to gene amplification,23,24 is activated through the binding of dephosphorylated Cdh1 to APC/C however, other potential causes have not been investigated yet. There in late M phase, and the continued absence of Cdk1 and Cdk2 is accumulating evidence that aberrant protein degradation of cell kinase activity keeps APC/CCdh1 functional throughout G1 (or G0). cycle regulators is an important factor contributing to tumorigeneAt the G1-S transition APC/CCdh1 function is blocked through both sis,25 and this may hold true for Skp2 (and Cks1) as well (see Fig. 1). the interaction of Cdh1 with the inhibitory protein Emi1 and Indeed, Skp2 stabilization has been observed in aggressive oral CDK-mediated phosphorylation of Cdh1.21,22 The inactivation of cancers (Kudo Y, Pagano M, Takata T, unpublished results). APC/CCdh1 permits the accumulation of Skp2 and Cks1, the assembly Increased protein levels of Skp2 in cancer cells may be a consequence of an active SCFSkp2 complex and the targeting of p27 and p21 for of inactive Cdh1 (e.g., due to mutations or overexpression of Emi1) or reduced Cdh1 expression. No data concerning the Cdh1 status in ubiquitin-mediated degradation. What are the consequences of defective Skp2 degradation in G1 cancer cells are available at present, but downregulation of Cdh1 has cells? Our experiments have shown that a nondegradable Skp2 been shown to accelerate cell cycle progression, a potential cause of mutant expressed at physiological levels in G1 cells promotes the genetic instability.17,18,26 It is also possible that alterations in other unscheduled degradation of p27 and p21. As a consequence, these components of the APC/CCdh1 complex contribute to Skp2-Cks1 cells progressed significantly faster into S phase than control cells. In stabilization. Indeed, it has been shown that in human colon cancer this context, it should be noted that the expression of the stable Skp2 cells two subunits of the APC/C, Apc6/Cdc16 and Apc8/Cdc23, www.landesbioscience.com Cell Cycle e28 HOW APC/CCDH1 KEEPS SCFSKP2 IN CHECK 25. Bashir T, Pagano M. Aberrant ubiquitin-mediated proteolysis of cell cycle regulatory proteins and oncogenesis. Adv Cancer Res 2003; 88:101-44. 26. Sorensen CS, Lukas C, Kramer ER, Peters JM, Bartek J, Lukas J. Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis. Mol Cell Biol 2000; 20:7613-23. 27. Wang Q, Moyret-Lalle C, Couzon F, Surbiguet-Clippe C, Saurin JC, Lorca T, et al. Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene 2003; 22:1486-90. te which are known to be key function elements in the complex, often display inactivating mutations.27 Alternatively, Skp2 may harbor mutations that obstruct any physical interaction between Skp2 and APC/CCdh1. Conceivably, such mutations would primarily affect the N-terminal D box or the Cdh1 binding site, rendering Skp2 refractory to APC/CCdh1-mediated ubiquitination and destruction. Future studies will therefore have to unravel how APC/CCdh1 targets Skp2 and Cks1 mechanistically to fully understand the role of defective Skp2/Cks1 degradation in cancer. © 20 04 La nd es Bio sci en ce. Do No tD ist r ibu References 1. Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev 1999; 13:1501-12. 2. Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev 1999; 13:1181-9. 3. Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 2003; 278:25752-7. 4. Carrano AC, Eytan E, Hershko A, Pagano M. Skp2 is required for ubiquitin-mediated degradation of the Cdk inhibitor p27. Nat Cell Biol 1999; 1:193-9. 5. Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol 1999; 1:207-14. 6. Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr Biol 1999; 9:661-4. 7. Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, et al. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol 2001; 3:321-4. 8. Spruck CH, Strohmaier H, Watson M, Smith AP, Ryan A, Krek W, et al. A CDK-independent function of mammalian Cks1: Targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell 2001; 7:639-50. 9. Sitry D, Seeliger MA, Ko TK, Ganoth D, Breward SE, Itzhaki LS, et al.Three different binding sites of Cks1 are required for p27-ubiquitin ligation. J Biol Chem 2002; 277:42233-40. 10. Seeliger MA, Breward SE, Friedler A, Schon A, Itzhaki LS. Cooperative organization in a macromolecular complex. Nat Struct Biol 2003; 10:718-24. 11. Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol 2003; 13:41-7. 12. Philipp-Staheli J, Payne SR, Kemp CJ. p27Kip1: Regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp Cell Res 2001; 264:148-68. 13. Pagano M, Benmaamar R. When protein destruction runs amok, malignancy is on the loose. Cancer Cell 2003; 4:251-6. 14. Masuda TA, Inoue H, Nishida K, Sonoda H, Yoshikawa Y, Kakeji Y, et al. Cyclin-dependent kinase subunit 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res 2003; 9:5693-8. 15. Shapira M, Ben-Izhak O, Bishara B, Futerman B, Minkov I, Krausz MM, et al. Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer 2004; 100:1615-21. 16. Wirbelauer C, Sutterluty H, Blondel M, Gstaiger M, Peter M, Reymond F, et al. The F-box protein Skp2 is a ubiquitylation target of a Cul1-based core ubiquitin ligase complex: Evidence for a role of Cul1 in the suppression of Skp2 expression in quiescent fibroblasts. EMBO J 2000; 19:5362-75. 17. Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCFSkp2-Cks1 ubiquitin ligase by the APC/CCdh1 ubiquitin ligase. Nature 2004; 428:190-3. 18. Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin Jr WG. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 2004; 428:194-8. 19. Yamano H, Gannon J, Mahbubani H, Hunt T. Cell cycle-regulated recognition of the destruction box of cyclin B by the APC/C in Xenopus egg extracts. Mol Cell 2004; 13:137-47. 20. Harper JW, Burton JL, Solomon MJ. The anaphase-promoting complex: It’s not just for mitosis any more. Genes Dev 2002; 16:2179-206. 21. Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nat Cell Biol 2002; 4:358-66. 22. Lukas C, Sorensen CS, Kramer E, Santoni-Rugiu E, Lindeneg C, Peters JM, et al. Accumulation of cyclin B1 requires E2F and cyclin A-dependent rearrangement of the anaphase-promoting complex. Nature 1999; 401:815-18. 23. Yokoi S, Yasui K, Saito-Ohara F, Koshikawa K, Iizasa T, Fujisawa T, et al. A novel target gene, SKP2, within the 5p13 amplicon that is frequently detected in small cell lung cancers. Am J Pathol 2002; 161:207-16. 24. Zhu CQ, Blackhall FH, Pintilie M, Iyengar P, Liu N, Ho J, et al. Skp2 gene copy number aberrations are common in nonsmall cell lung carcinoma, and its overexpression in tumors with ras mutation is a poor prognostic marker. Clin Cancer Res 2004; 10:1984-91. e29 Cell Cycle 2004; Vol. 3 Issue 7