Neurophysiol Clin 2000 ; 30 : 263-88
© 2000 Éditions scientifiques et médicales Elsevier SAS. All rights reserved
S0987705300002276/FLA
www.elsevier.fr/direct/nc-cn
REVIEW
Functional imaging of brain responses to pain. A review
and meta-analysis (2000)
R. Peyron1,2,3,4*, B. Laurent1,2,4, L. García-Larrea3,4
1
Département de neurologie, hôpital de Bellevue, boulevard Pasteur, 42055 Saint-Étienne, France ; 2 Centre de la
douleur, hôpital de Bellevue, boulevard Pasteur, 42055 Saint-Étienne, France ; 3 CERMEP, hôpital neurocardiologique,
59, boulevard Pinel, 69003 Lyon, France ; 4 UPRES EA 1880, université Claude-Bernard, Lyon, France
(Received 7 July 1999; accepted in revised form 19 May 2000)
Summary – Brain responses to pain, assessed through positron emission tomography (PET) and functional magnetic
resonance imaging (fMRI) are reviewed. Functional activation of brain regions are thought to be reflected by increases
in the regional cerebral blood flow (rCBF) in PET studies, and in the blood oxygen level dependent (BOLD) signal in fMRI.
rCBF increases to noxious stimuli are almost constantly observed in second somatic (SII) and insular regions, and in the
anterior cingulate cortex (ACC), and with slightly less consistency in the contralateral thalamus and the primary somatic
area (SI). Activation of the lateral thalamus, SI, SII and insula are thought to be related to the sensory-discriminative
aspects of pain processing. SI is activated in roughly half of the studies, and the probability of obtaining SI activation
appears related to the total amount of body surface stimulated (spatial summation) and probably also by temporal
summation and attention to the stimulus. In a number of studies, the thalamic response was bilateral, probably reflecting
generalised arousal in reaction to pain. ACC does not seem to be involved in coding stimulus intensity or location but
appears to participate in both the affective and attentional concomitants of pain sensation, as well as in response
selection. ACC subdivisions activated by painful stimuli partially overlap those activated in orienting and target detection
tasks, but are distinct from those activated in tests involving sustained attention (Stroop, etc.). In addition to ACC,
increased blood flow in the posterior parietal and prefrontal cortices is thought to reflect attentional and memory
networks activated by noxious stimulation. Less noted but frequent activation concerns motor-related areas such as the
striatum, cerebellum and supplementary motor area, as well as regions involved in pain control such as the
periaqueductal grey. In patients, chronic spontaneous pain is associated with decreased resting rCBF in contralateral
thalamus, which may be reverted by analgesic procedures. Abnormal pain evoked by innocuous stimuli (allodynia) has
been associated with amplification of the thalamic, insular and SII responses, concomitant to a paradoxical CBF
decrease in ACC. It is argued that imaging studies of allodynia should be encouraged in order to understand central
reorganisations leading to abnormal cortical pain processing. A number of brain areas activated by acute pain,
particularly the thalamus and anterior cingulate, also show increases in rCBF during analgesic procedures. Taken
together, these data suggest that hemodynamic responses to pain reflect simultaneously the sensory, cognitive and
affective dimensions of pain, and that the same structure may both respond to pain and participate in pain control. The
precise biochemical nature of these mechanisms remains to be investigated. © 2000 Éditions scientifiques et
médicales Elsevier SAS
allodynia / analgesia / attention / central pain / fMRI / imaging / motor cortex stimulation / nociception / PET
*Correspondence and reprints.
264
Peyron et al.
Résumé – Appréciation par l’imagerie fonctionnelle des réponses cérébrales à la douleur. Revue et méta-analyse.
Cette revue de la littérature concerne les réponses cérébrales à la douleur appréciées par l’imagerie fonctionnelle, soit
la tomographie d’émission de positons (TEP), soit l’imagerie par résonance magnétique fonctionnelle (IRMf). La
première mesure les variations de débit sanguin cérébral, la seconde les variations du signal BOLD (blood oxygen level
dependent) entre deux conditions. Pour l’étude de la nociception, la douleur induite par un stimulus nocif comparée à
un stimulus non nocif (en dessous du seuil) s’accompagne d’une augmentation presque constante du débit sanguin
cérébral et du signal BOLD dans le cortex insulaire, l’aire SII, et le gyrus cingulaire antérieur, de façon plus inconstante
dans le thalamus et l’aire SI. Les réponses insulaire/SII, thalamiques et SI sont supposées refléter l’aspect discriminatif
de la douleur. La réponse du cortex SI présente dans approximativement la moitié des études, apparaît liée à la surface
cutanée stimulée par unité de temps, elle semble donc dépendante des sommations temporelles et spatiales; elle est
modulée par l’attention portée au stimulus. La réponse thalamique, souvent bilatérale fait probablement intervenir des
phénomènes attentionnels d’« éveil » en réponse à la douleur. La réponse cingulaire antérieure (aire de Brodmann 24
et 32) ne participe vraisemblablement pas au codage de l’intensité du stimulus ni de sa localisation mais reflète
certainement des processus attentionnels et émotionnels associés à la perception douloureuse. Au sein de cette
structure, on distingue d’ailleurs plusieurs sub-divisions, l’une se superposant partiellement avec les activités
d’orientation et de détection de cibles, l’autre, plus antérieure et rostrale, correspondant plutôt à une attention soutenue
(exemple : Stroop, etc.). En plus de l’augmentation de débit cingulaire, l’attention au stimulus s’accompagne d’une
activité du cortex pariétal postérieur (aire de Brodman, BA 40) et du cortex pré-frontal dorsolatéral (BA 44 à 46) droits
qui participent au réseau cortical attentionnel et/ou mnésique. Les activations du striatum, du cervelet, de l’aire motrice
supplémentaire, moins commentées, pourraient intervenir dans la réponse motrice à la douleur, l’activation périaqueducale pouvant être impliquée dans les contrôles inhibiteurs descendants. Chez les patients, la douleur spontanée
s’accompagne d’une diminution du débit thalamique controlatéral, situation réversible sous thérapeutique analgésique.
L’allodynie, douleur évoquée par un stimulus non nocif, est associée à une amplification des réponses thalamiques,
insulaires et de SII, alors que la réponse cingulaire rostrale est diminuée, traduisant des anomalies de réorganisations
centrales postlésionnelles. Enfin, il apparaît que des procédures antalgiques, pharmacologiques ou neurochirurgicales,
augmentent le débit sanguin cérébral dans les mêmes régions que celles activées par la douleur aiguë, en particulier le
gyrus cingulaire antérieur et le thalamus. Ces données suggèrent que les réponses cérébrales à la douleur reflètent à la
fois les aspects sensoriel, cognitif et peut-être motivationnel de la perception douloureuse, et qu‘une même structure
peut à la fois répondre à la douleur et participer à son contrôle, même si la médiation biochimique de ces activités reste
à inventorier. © 2000 Éditions scientifiques et médicales Elsevier SAS
allodynie / analgésique / attention / douleur spontanée / imagerie / IRMf / nociception / stimulation de l’aire
motrice / TEP
The functional anatomy of pain in humans has been, in
recent years, mainly studied with positron emission
tomography (PET). This technique measures concentrations of isotopes within a given body volume; such
isotopes are carried by natural molecules which are
usually injected and enter the brain via the blood
stream. The physical variable that is directly measured
by PET cameras is therefore the distribution of radioactivity, while the associated physiological variable depends on the molecule that carries the positronemitting isotope. In studies where relatively rapid
changes in activity are to be measured, isotopes with a
short half-life are preferred, which allow repeated measurements in short amounts of time. One of the choice
isotopes is 15O, with a half-life of about 2 min only,
which can be included in natural molecules such as
water or butanol and yields information on regional
cerebral blood flow (rCBF). The so-called ‘activation’
PET studies investigate variations of rCBF specifically
associated to a given task or a particular stimulus. Data
interpretation is based on statistical comparisons of
rCBF values obtained in two clinical or experimental
situations, often labelled ‘activated’ and ‘control’ conditions.
PET has been applied to the study of pain since the
beginning of the 1990s, mainly by comparing responses to noxious and non-noxious stimuli, and has
brought relevant information to the understanding of
the ‘normal’ brain processing of pain. The insights
provided by the study of normal subjects have opened a
Brain responses to pain
large field of investigation in patients with chronic pain,
with the aim to understand (and possibly to treat) the
brain dysfunctions and reorganisations leading to these
conditions. The finding that the hemodynamic brain
response to pain is modulated by both cognitive [18,
122] and affective components [134] has joined electrophysiological data [66, 143], and connected with the
view of Melzack and others [101, 102], who described
pain sensation as the result of a multi-dimensional
integration of sensory-discriminative, cognitive, and
affective-motivational axes. However, this evolution of
pain imaging complicates the interpretation of new
data. Particularly, for each of the responses classically
described as ‘pain-related’, the question arises now as to
whether it is associated with the encoding of the sensory
(intensity, location, modality), affective (fear, unpleasantness), and/or cognitive (attention, memory) aspects
of pain integration, all of which contribute to the pain
experience. This view is further complicated by the
likely contribution of responses not linked to pain
integration per se, but related to preparation or inhibition of motor responses triggered by painful stimuli
[23]. Thus, the interpretation of imaging studies on
pain has moved from a ‘locationist’ conception to the
more fluid view of composite networks, where the
interaction of interdependent processes creates the unpleasant experience that we name ‘pain’.
In this overview we attempt to critically synthesize a
number of results, sometimes convergent and sometimes contradictory, obtained with PET and functionnal MRI (fMRI) during the past ten years. The review
will concentrate first on the responses to ‘laboratory’
pain observed in normal subjects. It will then comment
on the results obtained from patients suffering from
pain-related conditions and/or subject to therapeutic
procedures for pain relief.
SOME METHODOLOGICAL CONSIDERATIONS
OF PAIN IMAGING
CBF studies using PET
Even though the physiological significance of rCBF
changes with regard to neural activity is not clearly
established, there is considerable evidence that local
CBF changes are generated by metabolic products of
synaptic function, and therefore reflect variations in
local synaptic activity [147, 148]. The short scan duration (50–120 s) and inter-scan interval (10–15 min)
265
permit multiple studies in rapid succession, therefore
allowing comparisons between consecutive functional
states, including the resting state. The interpretation of
results is usually based on voxel-by-voxel subtraction of
images, looking for areas where rCBF is significantly
changed across conditions. In most pain experiments,
comparisons have been performed between two intensities of a thermal stimulus, one below and one above
the pain threshold, so that the subtraction analysis
extracts activity which can be ‘specifically’ attributed to
nociceptive processing.
Limitations of PET studies are :
– low temporal resolution due to signal averaging during approximately 1 min;
– the need of group analysis pooling the data of at least
five to six subjects to obtain meaningful results;
– the need for a nearby cyclotron facility to prepare
radioactive tracers;
– the need to give intravenous injections to the subjects.
These drawbacks may be partly overcome in the
future by fMRI, which uses similar experimental procedures as PET with a better temporal resolution, a
non-radioactive environment, no injection, and a possibility for individual analysis.
Studies using fMRI
Analysis of fMRI images is based on changes in the
blood oxygenation level dependent (BOLD) signal,
which reflects simultaneously local CBF changes and
variations in deoxyhemoglobin content [140, 158].
Results obtained with fMRI have been found to be
strongly correlated with those from PET-CBF in identical paradigms [32, 139, 142]. fMRI has some advantages over PET, including the operation in a nonradioactive environment and thus the possibility to
repeat recordings. Even though new-generation PET
cameras have been recently applied to single-subject
analysis [25], the possibility to take into account
anatomy and other individual characteristics by fMRI
is a clear advantage over PET. Furthermore, the access
to single-subject analysis will be an important gain in
pain studies, since pain is notoriously dependent upon
individual factors. Finally, the temporal resolution of
fMRI, which ranges from a 300 ms theoretical value to
a more realistic figure of 1–3 s in event-related fMRI
studies with echo-planar systems [15], is another advantage compared to PET; fMRI appears therefore as
266
Peyron et al.
an intermediate solution between PET resolution (tens
of seconds) and electrophysiology (tens of milliseconds).
Among the fMRI’s current drawbacks we should first
cite the requirement of MRI-compatible (i.e., nonferro-magnetic) equipment, as well as the need for strict
timing between stimuli and acquisition in rapidly alternating conditions, all of which add technical constraints, making some experiments more difficult to
conduct than with PET. In addition, a disadvantage of
fMRI is the existence of pulsation artifacts, which currently impairs analysis of brainstem and thalamic responses. More important, fMRI remains currently limited to ‘activation’ studies, and is neither able to provide
information on the resting state nor on neurotransmitters or receptors; this may represent a shortcoming in
future studies on pain, which should develop strategies
to describe the in vivo distribution and the functional
properties of neurotransmitters related to pain processing and control [86, 87, 161]. So far, the ‘pain responses’ obtained using PET and fMRI methods have
been very similar, but the use of this latter is still too
recent to permit definite conclusions, at least until a
comparison of results using both techniques in the
same population of subjects is available.
RESPONSES TO ACUTE PAIN IN NORMAL
VOLUNTEERS
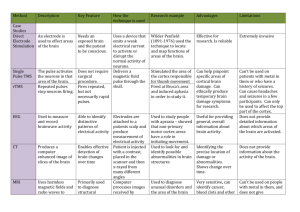
Table I summarises the results of previously published
PET studies in normal subjects. In a decreasing order of
consistency, hemodynamic responses to acute pain in
normal subjects have been observed in the following
brain areas: insular and SII cortices (primarily contralateral to stimulation but also ipsilateral); anterior
cingulate cortex (ACC, Brodmann areas [BA] 24 and
32); thalamus (primarily contralateral to stimulation
but often bilateral); SI cortex contralateral to stimulation; prefrontal (BAs 10 and 45–47) and posterior
parietal (BA 40) cortices; striatum; cerebellum (vermis); periaqueductal grey (PAG); and supplementary
motor area (SMA, BA 6). In figure 1, reported sites of
maximally significant rCBF increases to pain have been
projected onto a normalised MRI matching the Talairach and Tournoux atlas [152].
Insula/SII cortices
The most reliable pain-related activity across previous
studies is bilateral, and has been located in a broad
region comprising the depth of the Sylvian fissure and
the parietal and frontal operculi, and therefore extending from the anterior insula to the second somatic (SII)
area and associative parietal cortex (table I). It is not
easy to individualise these different regions from stereotaxic coordinates provided in previous PET studies,
since significant rCBF increases often extend over both
sagittal and vertical axes in adjacent brain regions. In a
number of studies, activations are reported as a focus of
increased CBF, which overlaps on both posterior insular and SII cortices [24, 27, 41], while in other cases two
separate foci of maximal significance are observed
within a large ‘activated’ area [22, 116, 150, 165]. This
is illustrated in figure 2, which also shows that the
location of activation maxima in the vertical axis ranges
from z = –8 mm to z = +32 mm when projected onto
the Talairach atlas. The particular anatomy of this
region, with numerous cortical folds increasing the risk
for partial volume effect, as well as the lack of a clear-cut
anatomical delineation of SII cortex in primates and its
known variability across individuals [97], are major
limiting factors to discriminate among different anatomic regions when using group analysis. In accordance
with the well-known bilaterality of SII and insular
receptive fields in both human [63] and animal [167]
investigations, more than 50% of imaging studies have
described a bilateral distribution of increased CBF in
insular/SII cortices during painful stimuli [5, 8, 10, 22,
24, 27, 29, 81, 99, 116, 122, 151, 156, 162, 165].
Notwithstanding these difficulties, analysis of figure 2
shows that in a significant proportion of cases, two
spatially distinct foci of maximal rCBF increases were
detected, one focus being antero-inferior, near the anterior insula, and the other postero-superior, in a region
corresponding to the retro-insular/SII interface. Spatial
discrimination of these two foci will probably become
accessible with the use of fMRI techniques (see recent
studies [7, 8, 10, 39]). However, even if the two foci
may be spatially discriminated, activation of SII and
insula seems, despite the functional differences between
the two areas, very often concomitant in response to a
noxious stimulus (see table I).
Previous literature suggests that neither anterior insular nor SII/retro-insular responses are specific for
pain, as they have also been described in response to a
wide variety of innocuous somatic stimuli, including
tactile [7, 81], electrical [62, 97], vibratory [27], innocuous thermal [7, 34] and olfacto-gustatory [56,
146]. Although these regions may therefore be involved
in general somatosensory integration, in the context of
Brain responses to pain
267
Figure 1. Summary of the principal regions showing maximally significant rCBF changes to painful stimuli in previous literature. Thirteen studies
providing stereotaxic coordinates of rCBF changes according to the Talairach and Tournoux atlas [152] are represented. The provided coordinates
of maximal rCBF increases in each study (volume of 8 mm3) were projected onto a normalised magnetic resonance template (MNI, Montreal
Neurologic Institute). Coordinates from studies using right-sided stimuli were taken just so, while those from studies using left-sided stimuli were
flipped along the x-axis; thus, the left and right sides of the figure are respectively ‘contralateral’ and ‘ipsilateral’ to stimulation. rCBF changes are
clustered in three midline regions (cerebellum/midbrain, thalamus, and anterior cingulate) and two lateral sites (around SII and the insular cortex).
For more exhaustive details on insular, SII, and cingulate activations, see figures 2, 3 and 5 (represented data are from references [22, 24, 27,
34, 42, 43, 81, 99, 122, 134, 150, 162, 165]).
Figure 2. Regional peaks of pain-related CBF increase in the insular and SII cortices across 34 studies. Peaks of maximal increase have been
plotted (when stereotaxic coordinates were available) onto axial brain slices from the Talairach and Tournoux atlas [152]. rCBF responses in
normal subjects are represented by red letters, while experimental (i) and clinical (p, s) allodynia as well as ongoing neuropathic pain (h) are
displayed in green for comparison purposes. Although regions of maximal response are widely scattered, most studies found two distinct peaks
of maximal response (letters represented twice). One peak was generally consistent with anterior insula (in upper row) and the other with posterior
insula/SII cortex (lower row). See also text for details. Note that sites activated during noxious stimulation and allodynia are very similar. Red
letters: b: see reference [8]; c: [24]; e: [43]; h: [75]; h: [74]; i: [81]; j: [41]; k: [27]; l: [28]; m: [99]; o: [116]; p: [122]; q: [34]; r: [134]; s: [150];
s: [151]; t: [153]; t: [156]; v: [162]; w: [10]; x: [165]. Green letters: h: [76]; i: [81]; p: [121]; s: [118].
Authors
PET
fMRI
N
Stimuli
T/P
Talbot et al.
1991
PET
8
Heat thermode
P
C
+
R
Jones at al.
Derbyshire et al.
Casey et al.
1991
1994
1994
1996
PET
PET
PET
PET
6
Heat thermode
6
Heat thermode
9
Heat thermode
9
Heat thermode
9 36-43°C Discrimination
9
Cold water bath
9
Heat thermode
16
Heat thermode
10 Sub-cutaneous histamine
4
Sub-cutaneous ethanol
11
Heat thermode
Cold thermode
Grill illusion of pain
7
Heat thermode
8
Heat water bath
12
laser
6
laser
11
laser
11 Electrical muscular pain
10
Heat thermode
9
Heat thermode
9
Heat thermode
6
Capsaicin injection
8
Esophageal distention
5
Esophageal distension
6
Visceral (rectal) pain
6
Visceral (rectal) pain
12
Heat water bath
P
P
P
P
P
T
P
P
T
T
T
T
T
P
T
P
P
P
T
T
P
T
T
P
T+P
T
P
T
D
D
C
C
C
C
D
C
C
C
C
C
C
D
C
D
D
D
C
C
D
C
C
D
C/D
D
D
C
–
–
+
+
+
–
+
+
–
–
–
–
–
–
–
–
+
+
–
–
–
–
–
–
–
–
–
–
R
R
L
L
L
L
L
R
R
R
R
R
R
L
L
R
L
L
L
R
R
L
R
R/L
R/L
R/L
R/L
R
13 Capsaicin injection (pain)
Capsaicin-induced
allodynia
9
Capsaicin-induced
allodynia
7
Capsaicin injection
(facial)
10
Heat thermode
M
10
Heat thermode
F
12
Heat thermode
12
Heat thermode
(intensity)
Heat thermode
(attention)
12
Heat thermode
T
P
C
C
–
+
P
C
T
Coghill et al.
1994
1999
1994
1995
1996
PET
PET
PET
PET
PET
Vogt et al.
1996
Rainville et al.
1997
Derbyshire et al. 1997
Xu et al.
1997
Svensson et al. 1997a
PET
PET
PET
PET
PET
1997b
PET
Hsieh et al.
Craig et al.
Adler et al.
Andersson et al.
Aziz et al.
Binkofski et al.
Silverman et al.
Baciu et al.
Derbyshire and
Jones
Iadarola et al.
1997 PET
1997 PET
1997 PET
1998 fMRI
1997 PET
1999 fMRI
1998 PET
1998
PET
Baron et al.
1999 fMRI
May et al.
1998
PET
Paulson et al.
1998
PET
Tölle et al.
Peyron et al.
Becerra et al.
1999
1999
PET
PET
1999 fMRI
C/D
Move- Side
ment
Ant
Insula
↑C
↑C
↑C
↑B
↑B
↑B
↑R
↑B
↑C
↑C
↑C
↑B
↑C
↑C
↑B
↑C
↑C
↑B
↑B
SII
mid
ACC ThalaACC (rostral) mus
24/32
↑C
↑C
↑B
↑C
↑C
↑C
↑C
↑C
↑C
↑B
↑R
↑C
↑C
↑C
↑C
↑B
↑C
↑C
↑C
↑C
↑B
↑C
↑B
↑C
↑B
↑C
↑C
↑C
↑C
↑B
–
–
–
↑C
↑C
↑C
↑C
↑B
↑B
↑L
↑B
↑B
SI
DLPFC MPFC Parietal Amygdala SMA 6
10,
9, 10,
40
Hippocam44-47
32
pus
↑C
↑C
↑C
↑C
↑B
↑B
↑C
↑B
↑C
↑B
↑C
↑B
↑I
↑B
↑C
↑C
↑C
↑C
–
↑I
↑C
–
↑C
↑C
↑C
↑C
↑C
↑C
↑C
ss ↑ C
ss ↑ C
↑C
↑C
–
ss ↑ C
↑C
↑C
Midbrain
↑C
↑C
↑I
–
↑I
↑C
↓I
↑B
↑B
↑B
↑B
↑R
↑B
↑C
↓B
↓B
↑B
↑B
↑C
↓C
↑C
↑B
↑I
↑I
↑B
↑C
↑C
↑B
↑C
↓B
↑C
↑C
↑C
↑C
↑C
↑C
↑C
↑
↑
↑B
↑I
↑I
↑
↑
↑
↑
↑
↑C
↑
ss ↑ C
↑C
↑I
↑B
↑C
–
R
↑C
↑C
–
–
C
–
R
↑B
↑I
↓C
P
D
+
L
↑I
↑C
–
P
D
+
L
↑C
T+P
T+P
C
C
–
–
R
R/L
↑B
(*↑C)
↑B
↑B
↑B
↑B
–
↑B
(*↑C)
↑C
↑C
↓I
–
–
↓I
↑B
↑C
–
T+P
C
–
R/L
–
–
↑C
↑B
–
↑ I (R)
↑B
–
↑B
↑B
P
C
–
L
↑B
↑C
↑B
↑C
↑B
↑C
↓I
↑C
↑C
↑C
↑B
↑C
↑B
↑C
↑L
↑R
↑I
↑
↑
↑
↑
↑
↑
↑
↑B
↑I
↑I
↑L
↑R
↑I
↑R
↑
–
L
L
↑C
↑I
↑C
↑C
↑C
↑I
↑B
↑
↑C
↑L
Cerebellum
↑C
ss ↑ I
↑I
↑C
↑R
↑B
↑R
↑B
↑I
↑B
↑C
↑B
↑B
LN
↑B
↑I
↑
↑
↑
↑
↑C
↑
↑C
↑I
↑C
↑C
(*↑)
↑I
↑I
↑C
↓B
↑I
↑
↑
↑
T: Tonic; P: Phasic pain; C: Continuous; D: Intermittent stimulus; Stimulus displaced (+) or not (–) during experiment; R/L side of stimulation; ss: sub-significant but
discussed as relevant by authors; ↑ or ↓ of rCBF or BOLD signal contralateral (C) or ipsilateral (I) to stimulus; B indicates bilateral or poorly lateralized activations; * indicates
abnormal (excessive) responses.
Peyron et al.
Year
268
Table I. Normal subjects (Nociception - Capsaicin allodynia).
Brain responses to pain
thermal stimuli their activity dramatically increases
when intensity reaches painful ranges (see table I).
Thus, Casey et al. [22, 24] did not find a significant
insular/SII CBF increase during discrimination of two
non-noxious heat stimuli, while insula was activated by
both heat and cold and SII was activated by noxious
heat. In a recent PET study, both anterior insula and
SII rCBF gradually increased with thermal intensity
[29]. In another study which separated activities related
to intensity coding from those linked to attention,
specific encoding of thermal pain intensity appeared
related to anterior insular activation, bilaterally [122].
Thus, in the context of thermal pain processing, both
the anterior insular and the retro-insular/SII cortices
appear as functionally implicated in the discrimination
of stimulus intensity. This is in agreement with animal
studies in primates [167], suggesting that the insular
cortex may gradually encode for different intensities of
stimulation, as well as with recent human recordings
from SII and insular regions showing gradually incremental responses to increasingly intense laser stimuli
[64]. These results are also in line with the recent claim
of a specific thalamo-insular pathway for cold, including noxious cold, in primates [33] and in humans [40],
which cortical projections to the insular cortex could be
gradually activated by cold (including noxious cold)
stimulations [35].
However, there is very little evidence from the literature that points out insular activity as related to attention. In their description of the attentional network to
pain, Peyron et al. [122] did not find the insular cortex
to participate in brain regions involved in selective
attention, while it is a major region involved in thermal
discrimination. The question whether insular cortex is
involved in emotional processing cannot be simply
answered because of the lack of studies investigating
directly this aspect of insular response to pain. A first
argument for such a participation is the activation of
insular cortex in emotional tasks with negative affective
components such as stimulation by fearful faces [125],
emotional voices [108, 109], or aversive conditioning
[14]. A second argument is the observed changes in the
emotional dimension of pain after lesion of the insular
region, while the discriminative dimension of pain is
spared. In this disconnection syndrome known as
asymbolia for pain [9], the subcortical lesion has been
considered as disrupting sensory-limbic connections.
269
Anterior cingulate cortex (ACC)
The ACC follows closely the anterior insula in the rank
of most frequently reported sites of rCBF increase to
pain (table I). In most cases the ‘activated’ sites corresponded to the ‘mid-cingular’ region (area 24), but a
number of studies have also showed hemodynamic
activations and deactivations of more anterior (rostral)
perigenual cingulate portions (see table I).
ACC, localisation of stimuli and intensity coding
The activity of anterior cingulate units is not suitable to
encode sensory aspects such as stimulus location, because of very wide and overlapping receptor fields [51,
144]. Accordingly, animal studies and clinical data
indicated that cingulotomy, although decreasing the
affective response to noxious stimuli, preserves the ability to localise such stimuli [60, 144, 159].
Whether the ACC participates in the encoding of
stimulus intensity is more difficult to resolve: in animals, response characteristics of ACC ‘nociceptive’
units are very similar to those of medial and intralaminar thalamic nuclei from which they receive projections
[144], and which have shown some intensity coding for
mechanical, heat [79], and electrical [93] noxious
stimuli [17, 50]. In addition, anecdotal reports in humans have suggested partial loss of the ability to code
stimulus intensity after cingulotomy [154]. However,
processing of intensity coding in the human ACC has
not been supported so far by functional imaging studies. Casey et al. [24] did not observe significant cingulate activation during active discrimination between
two non-noxious heat intensities. Peyron et al. [122]
described a network of structures involved in noxious
intensity encoding, which did not include mid-ACC.
Using regression methods, Tölle et al. [156] did not
find any relationship between ACC blood flow and
stimulus intensity. Furthermore, in some pain studies,
ACC activation was shown to increase in the absence of
any real change in stimulus intensity. For instance,
Craig et al. [34] used a ‘thermal grill’ to induce pain by
employing a combination of two non-noxious stimuli
(alternated warm and cool bars). Anterior cingulate was
not activated by either stimulus in isolation, but
showed enhanced rCBF when they were applied in
combination, suggesting that ACC changes were not
related to actual stimulus intensity, but rather to the
subjective pain sensation. In the study of Peyron et al.
270
Peyron et al.
[122], increased ACC activation was observed without
any change in actual stimulus intensity, during a ‘distraction’ experiment entailing decreased pain sensation. We may therefore conclude that there has been,
up to now, no support from imaging studies favouring
a role of ACC in coding stimulus intensity.
ACC and the affective reaction to pain
It has been generally considered that the ACC response
to noxious stimuli reflects the ‘suffering’ component of
pain [83]. Vogt et al. [162] suggested that the affective
reaction associated with pain unpleasantness would be
principally integrated in the rostral (perigenual) sections of ACC (BA 32 and 25), whereas mid-cingulate
activation (the one most commonly seen in PET and
fMRI studies) would be instead associated with cognitive processes, especially response selection and motor
inhibition. The implication of rostral perigenual ACC
in emotional and affective reactions is supported by
experimental and clinical studies [6, 47, 53] and also by
recent imaging studies that have manipulated the emotional stimulus content [11, 158]. However, the dichotomy between a perigenual ‘emotional’ and a
middle ‘cognitive’ ACC has not been supported by
other imaging studies. Neither Rainville et al. [134] nor
Tölle et al. [156] found any relationship between the
perigenual ACC and the affective reaction to pain. In
an elegant experiment, the Montreal group [134]
modulated the affective component of pain using hypnotic suggestion and reported a linear relationship between subjective unpleasantness and CBF in the midACC, rather than in its perigenual portion. Tölle et al.
[156] found that pain unpleasantness correlated positively with CBF in the posterior sector of ACC. Also in
studies assessing the reaction to the unpleasant character of stimuli from other modalities, such as the facial
expression of disgust [108], frightful animals [61], unpleasant musical dissonance [12], or words with negative semantic content [68], the main increases in CBF
have been observed in the middle and posterior sections
of the ACC rather than in its perigenual portions (see
figure 3b, blue letters). In turn, CBF in perigenual
cingulate was found to change independently of affective reactions by Svensson et al. [151], who reported
rostral ACC activation by tonic, but not phasic heat, in
spite of a similar affective reaction to both, as judged by
both autonomic (heart rate) and subjective (unpleasantness) measures. Thus, encoding of affective and
mood responses seems relatively distributed within the
anterior cingulate and, according to functional imaging
studies, may implicate rostral but also middle and even
posterior ACC portions.
It is noteworthy that dramatic CBF increases have
been repeatedly observed in the rostral ACC of psychiatric patients with obsessive-compulsive disorders [13,
136], phobic anxiety [137], post-traumatic stress [138],
or mood disorders [53]. These data suggest that stress
and anxiety, rather than unpleasantness, might be the
subjective variables most closely associated to CBF
increase in this portion of ACC. This could partly
explain the paucity of activation sites of rostral ACC in
pain studies since, due to extensive training and habituation of the subjects to the experimental paradigm, the
stress and anxiety component is likely to be minimised
in normal subjects. Conversely, this component is
likely to persist in patients with clinical pain, in whom
hemodynamic abnormalities (usually CBF decrease)
have been repeatedly reported in this area (see below).
ACC and cognitive-attentional response to pain
The implication of the middle ACC region in cognitive
responses to pain has received direct support from
recent studies [38, 44, 122, 135]. Using a factorial
design to separate the attentional and discriminative
components of the pain response, Peyron et al. [122]
found that ACC was not a part of the ‘intensity coding’
network, but was activated as part of an ‘attentional
matrix’ also involving the posterior parietal and prefrontal cortices. This mid-cingulate activation, mainly
in BA 32, proved to be dependent on sustained attention toward the stimulus, and independent of whether
the stimulus was noxious or not (figure 3b, green ‘p’).
This ACC activity was spatially concordant with that
observed in other attentional or ‘cognitive’ studies requiring sustained attention in the absence of pain
(Stroop test, word generation, etc.) and which are illustrated in figure 3b (green letters). A second mid-ACC
activation was observed in Peyron et al.’s study, with a
more caudal and lower position than the previous one
(see figure 3b, red ‘p’). This second cingulate activity (in
BA 24) was spatially distinct from the one associated to
sustained attention, and was also independent of ‘intensity coding’ since it appeared while subjects were
diverted from pain and produced lower thermal intensity scores. This activity was assumed to reflect phasic
attentional shifts to the sudden irruption of painful
stimuli, and subjects indeed reported to have had their
attention phasically drawn by noxious heat, in what
Brain responses to pain
271
Figure 3. Upper panel (3a): Localisation of maximal cingulate rCBF changes, as reported in published imaging studies on pain (PET and fMRI)
during the 1991–1999 period. Reported peaks of maximal rCBF changes were plotted (when coordinates were available) onto a sagittal brain slice
(x = 4 mm) formatted according to the Talairach and Tournoux atlas [152]. Pain-related increases of rCBF are represented by red letters; rCBF
decreases by blue letters. Pain studies (red): a: see reference [1]; b: [8]; c: [24]; d: [42]; e: [43]; f: [44]; h: [75]; i: [81]; j: [41]; k: [27]; l: [28];
m: [99]; o: [116]; p: [122]; q: [34]; r: [133]; s: [150]; s: [151]; t: [153]; v: [162]; w: [10]; x: [156].
Lower panel (3b): Reported loci of maximal CBF increase in imaging studies which manipulated selective, sustained attention (green letters), or
emotion (blue-grey letters). Emotional manipulations consisted of increasing (intense blue) or decreasing (blue/grey) unpleasantness, or
inducing stress/anxiety (grey). The letters r, f, and p in both a red and green colour refer to reports where pain and attentional responses were
investigated within the same study. Although there is overlapping between clusters, cingulate ‘activation’ during selective sustained attention
tends to be anterior and rostral to that obtained in pain studies. ‘Attentional’ studies (green): a: [20], (Stroop); b: see reference [16] (Stroop); c:
[30] (visuospatial & divided attention); d: [96] (divided attention); f: [44] (Stroop); g: [68] (Stroop); k: [90] (go/no go (posterior) & response
selection [anterior]); m: [110] (cognitive anticipation); n: [111] (visuospatial attention & target detection); o: [112] (auditory & visual attention);
p: [122] (sustained attention to one hand); q: [114] (Stroop); r: [135] (hypnosis); t: [155] (stimulus-response compatibility task); w: [163]
(Stroop); x: [103] (target detection). ‘Emotional’ studies (intense blue) = increased unpleasantness f: see reference [61] (frightful animals); g:
[68] (sad Stroop); l: [11] (anger facial expression); m: [108] (fearful facial expression); r: [134] (increased thermal unpleasantness); t: [156]
(increased thermal unpleasantness); u: [14] (aversive trace conditioning); w: [163] (negative emotion with sad Stroop);
(blue/grey) = decreased unpleasantness r: [134] (decreased thermal unpleasantness); (grey/blue) = stress, anxiety, and mood. b: [13]
(obsession-compulsion); r1: [136] (obsession-compulsion symptoms); d: [53]; r2: [137] (phobic symptoms); r3: [138] (stress).
272
Peyron et al.
they described as an orienting reaction. Since this latter
activation of BA 24 was similar in location to those
reported in other pain studies where attention was not
controlled (figure 3b, red dots), it was suggested that at
least part of mid-ACC activation in pain studies may
reflect phasic orienting to the painful stimuli. Similar
conclusions have been reached by Tölle et al. [156]
(figure 3a, red ‘x’) who, using regression analysis, observed that mid-ACC CBF correlated positively with
pain threshold intensities, and concluded that it could
reflect attentional shifts toward stimuli which capture
attention. Also supporting this view are some recent
studies [103, 112] in which hemodynamic activation
was observed in a very similar mid-ACC region (BA 24)
in cases of detection of suddenly-appearing auditory or
visual targets (figure 3b). These attentional experiments
would share with pain studies the need to monitor the
possible occurrence of sudden inputs. Therefore, in
general terms we may conclude that two distinct midACC hemodynamic activations can be observed in pain
studies, both of which reflect the cognitive dimension
of pain experience. The commonest of them is located
below the intra-cingulate sulcus (BA 24) and appears to
reflect attentional shifts to the painful stimulus, while
the other, above the sulcus (BA 32), would appear only
in cases of sustained and voluntary directed attention to
the stimulated area.
ACC and motor response to pain
Preparation and/or inhibition of motor reactions are
also functional responses triggered by pain and ACC is
known to participate in response selection [47, 157],
motor learning [71, 88], and motor planning [47, 126].
Some of the less commented ‘activations’ in PET
experiments, such as those within the cerebellum, basal
ganglia, supplementary motor area, and motor cortex
(see table I) might be indeed considered as part of this
‘motor’ response to pain, as could also be the case, to
some extent, for the ACC. To date, no functional
imaging study has specifically investigated this particular aspect.
ACC and anticipation of pain
Both the perigenual ACC and medial prefrontal cortex
modify their activities during the seconds preceding the
arrival of a noxious stimulus, as previously observed
during anticipation of cognitive tasks [110]. Changes
described up to now are manifested either as a decreased
[128] or increased [78, 127] signal, but aversive conditioning is known to increase the BOLD signal in rostral
ACC [14]. It is difficult at this point to judge whether
such signal changes are specifically related to the internal representation of impending stimuli (anticipatory
processes per se), anger [11], or rather reflect anxiety
and stress, which have also been shown to modify
orbito-frontal and perigenual ACC activity [13, 136138].
ACC as a multi-integrative structure
Convergent evidence summarised in previous sections
suggests that ACC supports multiple functions as a
subject experiences pain. From previous paragraphs, it
may sometimes appear that different processing axes
(sustained attention, orienting, stress, unpleasantness)
are indeed represented in different subsections of the
ACC. However, on the one hand, studies on groups of
subjects do not adequately reflect the high spatial variability of individual responses. On the other hand,
when the whole significant areas (rather than the ‘maximal’ peak responses) are considered, substantial overlap
exists between ACC activations. Individual variability
was demonstrated by Vogt et al. [162], while close
proximity and partial overlapping of ACC activations
with different functional significance (i.e., unpleasantness sensation and hypnotic suggestion) was shown in
studies by Rainville et al. [134, 135]. Even at a neural
level, animal experiments have demonstrated that subpopulations of ACC neurons may respond in a similar
way to different experimental contexts. For instance, a
large proportion of ACC neurons labelled as ‘nociceptive’ has been found to respond also to pain anticipation, i.e., preceding actual stimulus delivery [93]. It is
very likely that multi-functionality of ACC units and
regions also exists in relation with human pain: single
ACC regions may be involved in several functional
networks, and their processing capacities with respect
to a given function are probably modulated by other
concomitant processes. Accordingly, ACC activation
has been demonstrated to vary with the learning of
non-motor tasks [133] as well as the learning of nociceptive stimulations, in such a way that ACC can be
activated in naive subjects for an unlearned pain but is
no longer activated with the practice of tasks, increased
performance, and learned pain [78]. Functional interactions and internal modulations within the ACC deserve, therefore, to be specifically investigated in future
studies on cortical pain imaging.
Brain responses to pain
Primary somatosensory (SI) cortex
Previous reports on rCBF changes to pain in the primary somatosensory cortex have been notoriously inconclusive. Thus, while a number of studies have described significant pain-related rCBF increases in SI, a
comparable number of reports using similar methods
have failed to do so. From the 30 experiments (from 24
studies) on somatic pain summarised in table I, significant SI ‘activation’ was observed in 15 (63% of cases),
and no significant change in the other nine (46%).
Thus, the contribution of SI to pain processing, as
revealed by PET/fMRI experiments, is much less consistent across studies than that of the second somatosensory (SII), insular and anterior cingulate regions.
Different hypotheses have been put forward to explain such inconsistent results. Derbyshire et al. [43]
found that moderate painful stimuli entailed contralateral SI activation, while stimuli just above the pain
threshold failed to do so. However, the intensity of the
painful stimulus does not appear by itself to play a
major role in SI rCBF changes, since in a number of
other studies on somatic pain, such intensity was
enough to produce moderate to strong pain, yet no
hemodynamic activation of SI was observed [85, 122,
150, 162, 165]. High intensity levels may, however,
play an indirect role by increasing the level of attention
toward the stimulus (see later).
Since SI rCBF enhancement is more frequent for
moving than for immobile stimuli (table II), the hypothesis was put forward that SI changes might depend
on activation of separate groups of nociceptors by moving stimuli [83, 85, 122, 162]. However, clear contralateral SI activity has also been observed when using
non-moving painful stimuli such as hot or cold water
baths [24, 49, 134], mechanical stimulations [36], subcutaneous injections [2, 74, 75, 81], or immobile thermodes [8, 34, 151]. Furthermore, in the case of moving
stimuli, SI activation was obtained by comparing painful to non-painful conditions, where the stimulus
movement was identical. Thus, stimulus movement per
se does not appear to be the discriminating criterion
between studies activating SI or not.
A much less explored feature that may prove relevant
for SI hemodynamic activation is the stimulus’ spatial
summation. In experiments using skin stimuli, this
variable should be proportional to the total skin area
stimulated [72, 132], which in turn is determined by
1) the intrinsic stimulus size; and 2) the repetitive
application of the stimulus over different body sites. A
273
majority of published imaging studies can be classified
as activating ‘large’ or ‘small’ body surfaces depending
on these parameters; table II was constructed following
these lines, as applied to 30 experiments from 24 studies that used noxious skin stimuli. ‘Large’ body area
experiments involved a contact thermode successively
applied to multiple skin sites (usually 6) [21, 23, 27, 29,
34, 35, 115, 152], as well as studies using water baths or
other stimuli covering the whole hand [24, 34, 41, 134]
or electrical shocks to a large nerve trunk [37]. Conversely, ‘small’ body surfaces were considered to be
stimulated in studies using either laser stimuli or point
electrical stimulators [43, 150, 165], as well as those
that used contact thermodes applied to one single skin
site [8, 42, 85, 122, 162]. As shown in table II, spatial
summation appears as a relevant variable influencing SI
rCBF, since a vast majority of studies involving large
skin surfaces also activated the contralateral SI, while
most experiments using small surfaces failed to do so
(χ2 = 7.08, corrected P = 0.02; Fisher exact test,
P = 0.01). The average estimated surface in studies
showing SI CBF increase was about 16,300 mm2,
relative to 6,400 mm2 in studies that did not describe SI
activation. Published data suggest therefore that, when
using painful stimuli applied on the skin, spatial summation is a crucial factor that increases the likelihood of
hemodynamic SI activation in PET studies. The fact
that a relatively important amount of SI surface needs
to be excited to obtain significant increases of rCBF is
consistent with both the very limited number of units
specifically responding to pain in this region [91] and
the rare evocation of pain during SI stimulation [117].
The amount of spatial summation cannot be easily
estimated in studies using subcutaneous injections
(capsaicin, histamine, ethanol [74, 75, 81, 99]) or
visceral distension with a balloon [4, 5, 10, 145], so that
these figures are obviously too limited to derive conclusions at this stage.
Some studies also suggest a possible role of temporal
summation as a determinant of SI CBF increase. For
example, data from Svensson et al. [151] reveal that for
an identical stimulating surface of about 2,000 mm2, a
continuous stimulus yields more important SI activation than a phasic one. However, pooled data from
table II suggests that, in general terms, continuous
stimulation (labelled C+) was less discriminating than
the stimulation area in determining CBF increase, and
therefore spatial, more than temporal summation, critically influenced the probability of SI CBF increase.
274
Peyron et al.
Table II. Relationships between surface of stimulation and SI activation*
Surface
SI activation
Small
Derbyshire et al., 1997
Laser (79 mm2)
Becerra et al., 1999
Heat thermode (900 mm2)
Large
M- CM- C+
YES
NO
Jones at al., 1991
Heat thermode (1250 mm2)
Derbyshire et al., 1994
Heat thermode (1250 mm2)
Vogt et al., 1996
Heat thermode (375 mm2)
Peyron et al., 1999a
Heat thermode (900 mm2)
Xu et al., 1997
Laser (50, 625 mm2)
Svensson et al., 1997a
Laser (79 mm2)
Svensson et al., 1997a
Electrical (10 mm)
M- CM- CM- C-
Talbot et al., 1991
Heat thermode (79, 2800 mm2)
Casey et al., 1994
Heat thermode (254, 1524 mm2)
Casey et al., 1996
Heat thermode (254, 1524 mm2)
Casey et al., 1996
Cold water bath (56000 mm2)
Coghill et al., 1994
Heat thermode (100, 2800 mm2)
Coghill et al., 1999
Heat thermode (79, 3600 mm2)
Craig et al., 1996
Heat thermode (28000 mm2)
Rainville et al., 1997
Hot water bath (56000 mm2)
Paulson et al., 1998 (males)
Heat thermode (254, 1524 mm2)
Davis et al., 1995
Electrical (median nerve, 28000 mm2)
Svensson et al., 1997b
Heat thermode (1256, 1600 mm2)
Craig et al., 1996 (ss)
Cold thermode (28000 mm2)
Derbyshire and Jones, 1998
Hot water bath (56000 mm2)
Paulson et al., 1998 (females)
Heat thermode (254, 1524 mm2)
Tölle et al., 1999
Heat thermode (2304 mm2)
M+ C+
M+ C+
M+ C+
M- C+
M+ CM+ C+
M- C+
M- C+
M+ CM- C+
M- C+
M- CM- C+
M- C+
M+ CM - C+
M- CM- C+
M- CM+ CM- C+
* First number in brackets indicates the surface of stimulus (thermode, laser beam, bath, etc.). The second number indicates, for stimuli
applied over multiple sites (M+), the total surface of stimulation (56000 mm2 is indicative of the surface for hand immersion in a water bath)
(It has been fixed by authors as approximately double of those used for the thermal grill applied on the palm). M- indicates that stimuli were
not displaced during PET or fMRI recordings; C+ indicates continuous stimuli while C- indicates intermittent stimuli; ss :sub-significant
activation discussed by authors as relevant.
Accordingly, spatial summation has been shown to
modify the cognitive, affective, but also the sensorydiscriminative dimension of pain appraisal [72, 113,
150]. From a pragmatic point of view, increasing the
total stimulated surface (and possibly also the total
stimulation time) may be the simplest way of increasing
the likelihood of SI activation in further imaging studies.
In addition to the mechanisms described earlier, converging evidence suggests that rCBF increase in SI may
Brain responses to pain
partially depend on attention directed to the painful
stimulus. Electrophysiological studies have shown that
selective attention enhances neural activity from SI,
both in animals [59, 82, 168] and in humans [46, 65,
106]. PET studies have also shown that attention directed to a tactile stimulus enhances both glucose consumption and the hemodynamic response in SI [69,
105]. Evidence that these mechanisms can be relevant
for pain experiments has been recently provided by
Bushnell and coworkers [18] in a study where the rCBF
increase in contralateral SI was enhanced as attention
was turned to a noxious stimulus. Based on stimulusresponse characteristics of SI neurons in monkeys, it
has been concluded that two different populations
within SI participate respectively to the sensory and
attentional aspects of pain processing [26, 59]. All these
results probably underlie the psychophysical observation that attention directed to a painful stimulus increases its detectability [107]. In general terms, it can be
hypothesised that, in the painful ranges, moving
stimuli are more likely to drive spatial attention than
immobile ones, and heavily supraliminal stimuli more
than stimuli barely at pain threshold. It is, however,
noted that neither attention nor high intensity alone
appear to be sufficient to ensure a PET-detectable rCBF
increase in SI in the absence of adequate spatial summation (see [124]).
In addition to increased rCBF in contralateral SI,
several groups have described decreased blood flow [3],
particularly in portions of the SI region that do not
correspond to the stimulated body area. Decreased
rCBF has been observed in SI ipsilateral to a noxious
stimulus [122], or in both contralateral and ipsilateral
SI regions corresponding to non-stimulated areas (for
instance, the leg and face representation in case of a
hand stimulus [52]). Since this phenomenon was observed even in the absence of actual stimulus, it has been
attributed in part to cognitive variables such as anticipation of pain [53] or focalised attention to the site
being stimulated. An rCBF decrease in sensory areas
that do not receive relevant input may represent an
‘economical’ brain mechanism to facilitate stimulus
detection by enhancing the contrast between regions
concerned or not by stimuli [53].
275
tion of these regions in both attentional and executive
functions is well known, their activation being frequently described in experiments involving attention,
working memory, and goal-directed processes [21, 31,
32, 57, 70, 92, 95, 100, 111, 115, 130, 166] (for a
review, see [104]). In the context of pain experiments,
both posterior parietal and DLPF activations are therefore likely to mediate part of the cognitive dimension of
pain processing associated with localisation and encoding of the attended stimulus [122]. Although bilateral,
these cortical activities often show asymmetrical distribution and predominate on the right hemisphere, regardless of the side of stimulation [41, 42, 85, 116, 122,
162], as has been previously observed in attentional
experiments [31, 70, 115].
Thalamus and brainstem
Thalamus has been often but inconstantly activated
across PET or fMRI studies on pain (table I). Thalamic
activation is frequently described as bilateral [23, 24,
44, 162], suggesting that it does not merely reflect a
sensory response, which would be supposed to predominate contralaterally to the noxious stimuli. Furthermore, attentional processes and vigilance have also
been shown to increase thalamic activity bilaterally [61,
111, 129-131] and thus, thalamic enhancement in pain
studies may also reflect a general ‘arousal’ reaction to
pain ([122], see figure 4). Thus, thalamic hemodynamic
responses to painful stimuli can be considered as a part
of both discriminative and attentional networks involved in pain processing.
The thalamus contains a great number of inhibitory
synaptic connections mainly involving the reticularis
thalami, and these may contribute to bilateral thalamic
and brainstem activations seen in pain studies. For
instance, brainstem activity in response to pain is commonly reported as corresponding to the periaqueductal
grey matter (PAG), but inspection of data usually
shows that it greatly exceeds this localisation, includes
reticular formation, and often appears as a caudal extension of thalamic activation. It may reflect synaptic
activation of mesencephalic nociceptive relays related
to arousing activity, to the set-up of descending pain
controls, or both.
Prefrontal and posterior parietal cortices
Dorso-lateral prefrontal (DLPF) and (to a lesser extent)
posterior parietal responses have been repeatedly reported as ‘pain-related’ activities (see table I). Participa-
Other brain regions
Among the several other areas that have shown hemodynamic changes to pain, the presence of brain regions
276
Peyron et al.
Figure 4. Different components of the brain haemodynamic response to pain according to Peyron et al. [122]. Subjects received low- or
high-intensity thermal stimuli while directing or not their attention to the stimulated hand. The ‘intensity coding’ component (top left) was obtained
by subtraction of ‘low intensity’ from ‘high intensity’ conditions, regardless of attention. The ‘attention component’ (bottom) was obtained by
subtraction of ‘no task’ from ‘attentional’ scans regardless of stimulus intensity. The ‘attentional component’ involved a large network including
prefrontal, posterior parietal, and cingulate cortices and thalami. This component may be tentatively divided into ‘arousal’ and ‘selective attention’
systems. Decreased rCBF in the primary sensory cortex ipsilateral to the painful stimuli might participate to contrast enhancement or reflect
anticipation of pain. For further details, see text and references.
involved in motor functions is noteworthy, particularly
the lenticular and caudate nuclei, the cerebellum (vermis and hemispheres) and the SMA. Even the primary
motor cortex has been found to respond with the rCBF
increase in some studies [5, 24, 151], and with the
rCBF decrease in others [122]. Whether the primary
motor area is activated independently of, or in conjunction with SI, and whether it reflects motor activation
(withdrawal reaction) or a motor inhibition (movement refrain) cannot be ascertained at this time.
BRAIN RESPONSES IN PATIENTS WITH PAIN
Spontaneous pain in patients with neuropathic
pain
Spontaneous pain is difficult to investigate using functional imaging due to the need to compare in the same
subjects a painful versus a pain-free condition. This
binary situation is rare in clinical practice and the
literature is therefore restricted to a few reports, in
Brain responses to pain
patients with either cancer pain alleviated by cordotomy [48], ongoing neuropathic pain alleviated by
anaesthetic blocks [76], or central pain treated with
motor cortex stimulation [67, 119]. One common
finding in these studies was a relative decrease of thalamic rCBF during ongoing pain, which receded after
analgesic treatment. In addition, a relative hypoperfusion of the thalamus contralateral to ongoing pain
(compared to the ipsilateral side) has been verified in
patients with either peripheral neuropathic pain [76,
80] or central pain after cortical lesions sparing the
thalamus [119, 123]. These findings suggest that ongoing neuropathic pain (central or peripheral) is often
linked to thalamic hypoperfusion, and that a variety of
analgesic treatments are mediated through an increase
in thalamic blood flow.
Provoked pain in patients with neuropathic pain
The term ‘allodynia’ refers to abnormal pain triggered
by a non-noxious stimulus (i.e., light touch, contact of
the skin, brushing, non-noxious cold). Allodynia reflects, therefore, a ‘misinterpretation’ of somatosensory
information, which abnormally evokes a painful experience for intensities clearly below the normal pain
threshold. In patients suffering from allodynia, this
symptom can be reproduced during PET or fMRI
sessions, and it is thus easier to explore than spontaneous pain (figure 5). The main limitation of ‘allodynia’
paradigms is that responses to allodynic stimuli cannot
be compared with innocuous stimulation of the same
territory, since any skin stimulation in the affected area
rapidly induces unbearable pain. The allodynic stimulation is therefore compared either to a ‘resting’ (no
stimulation) condition, or to an identical stimulation of
a non-affected body area. A study in patients with
allodynia after a lateral medullary infarct (Wallenberg’s
syndrome) showed that allodynic stimulation (light
rubbing of the affected area) induced both a pain sensation and brain activities which are usually associated
with pain processing, notably in the thalamus, anterior
insula, SII, and posterior parietal cortex, while such
activities were not observed when the same stimulus
was applied to the normal side [121] (figure 6). These
data were interpreted as reflecting abnormal stimulus
amplification in the thalamus and thalamo-parietal
loops, leading to increased rCBF in the ‘lateral’ discriminative pain system (i.e., lateral thalamus and parietal cortex), and activating attentional (posterior parietal) networks. A similar pattern of amplification of the
277
thalamo-parieto-insular response was described in normal subjects suffering from experimental allodynia after
injection of capsaicin [7, 81].
In contrast with this unequivocal thalamo-parietal
behaviour, allodynic responses in the ACC and medial
prefrontal regions seem to be more complex, and, as for
nociceptive pain, should be separated into mid-ACC
and rostral ACC activations. Mid-ACC have firstly
shown variability in the experimental model of
capsaicin-allodynia since a bilateral rCBF increase has
been observed in a first study [82] but has not been
confirmed in another one [7]. Secondly, increased activity of the mid-ACC has been reported in the two
studies on clinical allodynia after peripheral nerve lesions [76], while such activation was not observed in
patients with allodynia after a lateral medullary (Wallenberg’s) infarct [121]. Interestingly, and thirdly, in
these latter patients, investigation of the basal hemodynamic status showed that this mid-ACC region specifically had a ‘paradoxical’ decrease of rCBF [120], in a
localisation highly congruent with rCBF decreases reported in other non-neuropathic pain situations [see
later, 45, 84, 141]. Since the mid portion of ACC
receives numerous inputs from spino-thalamic tracts
[51, 144] and since different amounts of deafferentation can be observed in neuropathic pain patients (for
instance, patients with Wallenberg’s syndrome have a
pure spino-thalamic syndrome while those with peripheral nerve lesion had a less selective involvement),
deafferentation itself may participate in hemodynamic
results, independently of pain. Finally, it is noteworthy
that the most consistent ACC response to allodynia,
regardless of the level of the lesion (i.e., peripheral or
central), is a decrease of rCBF located in the rostral
portion of ACC [76, 121]. In the absence of more
numerous studies, it cannot yet be ascertained whether
these disparities are purely methodological in origin or
reflect genuine differences between experimental and
clinical allodynia. If these data are confirmed by further
studies, the ‘lessened’ reaction of the rostral ACC and
medial prefrontal cortex to allodynic stimuli might be
one characteristic of allodynia resulting from neuropathic lesions.
Other clinical pain situations
Apart from neuropathic pain, the mid-ACC portion
has also been pointed out as a major cortical target of
hemodynamic abnormalities in studies on clinical pain
situations without lesion on the neuraxis. Angina
278
Peyron et al.
Figure 5. Upper panel (5a): Loci of pain-related cingulate CBF increase in normal subjects (red spots) compared with those of patients with
clinical pain. Capital green and blue letters refer respectively to increased and decreased blood flow during clinical pain situations:
pharmacologically-induced cluster headache (H: [77]; M: [98]); nociceptive pain in atypical facial pain patients (D: [42]); dental pain patients (E:
[45]); pain from rheumatoid arthritis (J: [84]); ongoing neuropathic (peripheral) pain (N: [76], S: [118]); angina pectoris (R: [141]); allodynia (P:
[121]); and basal rCBF in patients with chronic central pain (W: [120]) after Wallenberg’s syndrome. Although most of the CBF changes in clinical
pain are located in areas which also respond to normal nociception, a decrease of CBF has often been reported in patients. Such paradoxical
responses might be relevant for the understanding of abnormal pain processing.
Lower panel (5b): Pain-related ACC responses in normal controls (red spots) and foci of increased CBF during analgesic procedures (blue letters),
using opioids (O: [58], O: [1]); anaesthetic blocks (A: [76]); spinal cord (S: [73] [decreased rCBF]); thalamus stimulation (T: [54]); or motor
cortex (M: [67], M: [123]).
pectoris [141], cluster headache [98], atypical facial
pain [42], dental pain [45], and pain from rheumatoid
arthritis [84] all demonstrated abnormal (i.e., increased
or decreased; see figure 5, table III) activity in the mid
and/or rostral portion of ACC. Hemodynamic abnormalities in a restricted area of the mid-ACC in patients
compared to normal subjects [42, 45, 84] lead some
authors to conclude that reduced ACC response to
acute pain may be one adaptive cortical mechanism
characteristic of patients with chronic pain. An alternative (or complementary) view comes from recent data
showing an rCBF decrease in rostral ACC and medial
prefrontal cortices during anticipation of a previously
learned pain [78]. The question arises as to whether
Brain responses to pain
279
Figure 6. The insular/SII responses to either nociception (top row) or allodynia (bottom row). Responses to nociception were obtained by PET
from 12 volunteers submitted to a thermal stimulation on the right hand [122]. Responses to allodynia were obtained from patients with
right-sided lesions and therefore a left allodynic pain. Note that insular/SII nociceptive responses to high intensity (painful) thermal stimuli in
normal subjects (top) are very similar to allodynic responses in patients. Note also that allodynic responses in insula/SII are obtained with a
low-intensity (normally non-painful) stimulus consisting of non-noxious cold rubbing on the left thigh (lower row): Left: Patient 1 had allodynia
secondary to an isolated SII lesion (fMRI, unpublished data). Middle: Patient 2 had a combined SI, SII, and anterior cingulate lesion (PET and fMRI
images, published as a single case, see [124]). Right: Nine patients with lateral medullary infarct (LMI) were studied as a group by PET (see
[121]).
anticipation of an intensely distressful and well-learned
sensation, rather than the sensation itself, might also
contribute to the blunted ACC response in allodynia.
Analgesic procedures
In that context, it may be of importance to note that
analgesic procedures, including administration of
opioids [1, 58] and neurostimulations for pain relief
[54, 67, 73, 119], all increased rCBF in the ACC (figure
5b, table IV). Particularly, opioids and stimulation of
both thalamus and motor cortex increased rCBF in the
rostral ACC and basal orbitofrontal cortices, at very
similar sites where it has been found to be decreased in
allodynic or chronic pain patients (figure 5a, b). These
convergent, although preliminary, data suggest, therefore, that regulation of activity at the
orbitofrontal/ACC regions may play a role in
stimulation-induced pain relief. Although the precise
participation of these areas in patients’ relief remains
unknown, their functional role in animals and humans
suggests that they might either contribute to normalise
stress, anticipatory and mood processes, the alteration
of which is common to different kinds of chronic
painful states, or activate descending inhibitory controls of pain.
GENERAL DISCUSSION AND FUTURE LINES
OF RESEARCH
Functional imaging and subcomponents
of the pain experience
Imaging studies in recent years have allowed the visualisation of a number of brain regions which consistently respond to pain with changes in blood flow.
These cortical targets appear to subserve different aspects of the multidimensional pain experience; thus,
the sensory-discriminative aspects of pain perception
appear to implicate the lateral thalamus, primary and
second somatosensory regions and the insular cortex,
280
Table III. Patients with chronic pain.
Disease / Authors
Atypical facial pain
Derbyshire et al.
Neuropathic (central) pain
Peyron et al.
Stimuli
T/P Move- Side Ant
ment
Insula
SII
↑C
mid ACC ThalaACC (ros- mus
24/32 tral)
↑Β
(*↑)
1994 PET
6
Heat thermode
P
–
R
1994 PET
12
Parmacological induction
T
–
R/L
1995 PET
5
Patients vs normals, rest
T
–
R/L
1995 PET
8
Ongoing pain vs relief
T
–
R/L
↑Β
1996 PET
7
Pharmacological induction
T
–
R/L
↑Β
↑R
1998a PET
9
Pharmacological induction
T
–
R/L
↑Β
↑R
1997 PET
1997 PET
1999 PET
6
6
6
Ongoing pain vs rest
Heat thermode
Heat thermode
T
P
P
–
–
–
R/L
R
R
↑Β
(*–)
(*↓)
(*↓)
1998 PET
9
Wallenberg’s syndromes
allodynia
Electrical pain
P
T
+
–
R/L
R/L
↑C
↑Β
↑C
–
Mononeuropathy
P
+
R/L
ss ↑ I
↑Β
Neuropathic (peripheral) pain
Petrovic et al.
1999 PET
5
↓Β
SI
DL- MPFC Parie- Amygdala
SMA
PFC 9, 10, tal 40 Hippocampus
6
10,
32
/7
44-47
↑
↑L
↓Ι
(*↓)
↑Β
↓C
↑Β
↓L
↑Β
↑Β
LN
Mid- Cerebrain bellum
↑
↑
↑
↓C
–
↑R
↑C
↓Β
↓C
–
↑Β
↑R
↓Β
↓L
↑R
↑R
↑Β
↑R
↑
(*–)
(*↓)
(*↑)
(*↑)
↓Ι
↑C
–
↑C
–
↓C
↑Β
↑C
↑ Ι (*↓)
(R)
(*↓ Ι)
↑C
↑Ι
↓C
↓Ι
↑C
↓ Ι (R)
↑C
(*↑)
↑Β
↑Β
↑C
↓Β
↑C
↑
↑
Peyron et al.
Angina pectoris
Rosen et al.
Neuropathic (peripheral) pain
Iadarola et al.
Neuropathic (peripheral) pain
Hsieh et al.
Cluster headache
Hsieh et al.
Cluster Headache
May et al.
Irritable bowel syndrome
Silverman et al.
Rheumatoid arthritis
Dental pain
Year Moda- N
lity
Table IV. Analgesic procedures studied with PET.
Disease / Authors
Modality
N
1991 PET
5
1995 PET
1999 PET
Stimuli
T/P Move- Side Ant
ment
Insula
T
–
R/L
T+P
–
R/L
2
10
Cancer pain
Cordotomy
Central pain
Stimulation
Stimulation
1998 PET
5
Neuropathic pain
T+P
–
1991 PET
1
T
–
Firestone et al.
1997 PET
6
Adler et al.
1997 PET
9
Cancer pain
Morphine analgesia
Fentanyl in normals
Heat pain
Fentanyl analgesia
1995 PET
8
Regional lidocaine blocks
Anaesthesic blocks
Hsieh et al.
SII
mid ACC ThalaACC (ros- mus
24/32 tral)
↑Ι
↑Β
↑Β
R/L
↑Ι
ss ↑ I
L
↑C
T
–
L
T
–
R/L
↓Β
↑Β
↑Β
↑I
↑Ι
(*↑)
↑Β
↑Β
↓Β
↑Β
↓C
↑C
↓C
↑C
↑C
SI
DL- MPFC Parie- Amygdala SMA
PFC 9, 10, tal 40 Hippocampus 6
10,
32
/7
44-47
↓C
LN
↑Β
↑Β
Mid- Cereebrain bellum
↑Β
↑C
ss ↑ I
↑C
↑I
↓Β
↑I
↑Ι
(*↑)
↑C
↓Β
↑Β
↑Β
↓Β
↑Β
Brain responses to pain
Anterior cordotomy
Di Piero et al.
Motor cortex stimulation
Peyron et al.
Garcia-Larrea et al.
Thalamic stimulation
Duncan et al.
Opioids analgesia
Jones et al.
Year
↑C
↑C
(*↑)
↓Β
T: Tonic; P: Phasic pain; Stimulus displaced (+) or not (–) during experiment; R/L side of stimulation; ss: sub-significant but discussed as relevant by authors; ↑ or ↓ of rCBF
or BOLD signal contralateral (C) or ipsilateral (I) to stimulus; B indicates bilateral or poorly lateralized activations; * indicates abnormal (excessive) responses.
281
282
Peyron et al.
while the additional activation of posterior parietal and
prefrontal cortices appears to subserve the cognitiveattentional processing of noxious information. Different subsections of the anterior cingulate cortex are
likely to underlie cognitive (orienting, response selection) and affective (aversive) reactions to pain; although
some discrimination among ACC subsections may be
done on the basis of meta-analyses (figure 3), it is still
premature to ascribe precise ACC subdivisions to welldefined cognitive operations or especially affective reactions. Regions implicated in pain inhibition (periaqueductal grey) and in motor control (basal ganglia, SMA,
cerebellum) also show inconstant rCBF increase during
painful stimulation, probably reflecting the setup of
descending inhibitory controls, as well as of motor and
pre-motor mechanisms linked to the avoidance reactions to pain [160].
As in every schematic classification, the above assertions deserve some nuance: for instance, there is little
doubt that both SI and SII cortices also participate in
the attentional processing of somatic stimuli, and that
thalamic activation (especially when bilateral) also reflects pain-induced generalised arousal. The affective
dimensions of the pain experience remain poorly investigated, probably because ‘laboratory pain’ is not a good
model for inducing intense affective reactions in trained
subjects [153]. It appears, however, that the hemodynamic correlates of ‘pain unpleasantness’ in normal
subjects and patients with chronic pain greatly differ:
while in normal controls increased unpleasantness correlates with enhanced rCBF in the mid-cingular or
posterior portions of the anterior cingulate cortex [134,
156], a rCBF decrease in more rostral areas of ACC
(BAs 32 and 10) has been reported in patients undergoing clinical, intensely unpleasant pain [141]. Further
studies aimed at modulating specifically the emotional
reactions to pain, both in healthy subjects and patients,
should in the near future ameliorate our insights into
these variables.
The subtraction and normalisation procedures in
brain imaging studies
Subtraction of ‘control’ images from those obtained
during test conditions reveals changes which are associated uniquely with the test condition. The subtraction procedure has evident advantages, in that it eliminates common sources of variance between conditions,
such as general anxiety or stress. Without elimination
of such data results would be much more difficult to
interpret. However, this methodology also entails limitations in data interpretation, and its use to study the
neural substrates of cognitive activities has been recently challenged [166]. Subtraction images do not
provide information about the complete network involved in stimulus processing; therefore, finding regions where the processing of innocuous and painful
stimuli is different does not imply that such regions are
sufficient to sustain the experience of pain. In turn, the
fact that a given area is not differentially activated by
innocuous and painful stimuli cannot discard its possible participation to pain processing. The activity of
such a region may have a different functional content
depending on whether or not it is associated with that
of other areas activated by noxious stimuli. Therefore,
the pain experience should not depend on the activity
of regions exclusively driven by noxious input, but
rather on the interaction between these regions and
other areas giving similar response to noxious and innocuous stimuli, which are eliminated by subtraction.
Normalisation of different conditions for global activity is also a common procedure that enhances focal
changes in rCBF and eliminates the need of arterial
sampling. However, this method also ignores global
CBF modifications that may prove relevant to our
understanding of pain processing by the brain. For
instance, it has been recently demonstrated that painful
stimulation may actually decrease global CBF by more
than 20%, relative to resting levels [28], which represents a previously unidentified response to pain. Experimental designs using several activation levels,
comparison between rCBF and electrophysiological
changes including those from intracranial electrodes,
and utilisation of event-related fMRI techniques are
among the procedures that might overcome these difficulties in the near future.
Functional significance of PET and fMRI signals
An everlasting problem associated to both PET and
fMRI results is the interpretation of blood flow changes
in terms of ‘activation’ of underlying cerebral structures. Substantial evidence supports that increased
rCBF reflects increased synaptic activity [147, 149],
which may reflect either activating or inhibitory
energy-consuming processes. The rate of blood flow
increase is determined by firing rates in the synaptic
terminals, and this whether an excitatory or an inhibitory neurotransmitter is released. To quote Sokoloff
[148]: “To distinguish between the two, one must look
Brain responses to pain
one synapse downstream; if an inhibitory transmitter is
released... one will observe reduced glucose utilisation
in the next synapse. If an excitatory neurotransmitter is
released, then glucose utilisation will increase at the
next synapse.” It is obviously impossible with current
PET or fMRI technology to ascertain rCBF changes “in
the next synapse” of a given region. Network analysis,
which seeks for statistical correlation of dynamic flow
changes between interconnected regions, is one promising strategy that might partially surmount this limitation [19, 89, 164] and may help significantly to interpret the functional significance of the observed
changes.
Neurochemical basis of pain-related imaging
A further field which deserves intensive investigation is
the neurochemical basis of pain-related hemodynamic
changes. Variations of rCBF associated with opioid
analgesia have been explored in a few studies with
rather congruent results (table IV, figure 5b). The
opioid agonist fentanyl increases rCBF in the rostral
part of anterior cingulate and orbito-frontal cortices in
normal subjects [1, 58]. This region is involved in pain
integration (see earlier) and contains a high density of
opioid receptors [86], the density of which is known to
change during pain states. For instance, in chronic
rheumatoid arthritis, the binding on opioid receptors is
decreased during inflammatory phases and pain relapses, and increased during remissions [87]. On the
other hand, rCBF was found to increase in very similar
locations during non-pharmacological analgesic procedures such as motor cortex and thalamic stimulation
[54, 67, 94, 119]. Thus, increased synaptic activity in
the rostral ACC/orbito-frontal boundary may be an
important common effect of both drug and neurostimulation analgesia, reflecting presumably increased
activity in pain control areas. Although this also suggests that part of the analgesia-related rCBF changes
might depend on opioid receptors, no direct proof of
this is currently available. It is likely that in vivo neuroreceptor mapping studies in coming years will be of
importance for the understanding of these issues and
therefore for the treatment of chronic pain.
CONCLUSION
This review has shown a good deal of convergent data
and promising results that should, in the next years,
improve our understanding of pain processing in the
283
brain. In spite of the remaining discrepancies and interpretive difficulties, analysis of the literature shows an
overall coherent picture of brain networks involved in
pain processing, in fact much more coherent than what
emerges from current meta-analyses in other fields of
brain imaging [55]. We believe that pain imaging studies are and will be helpful not only for the understanding of acute or chronic pain and pain-associated processes, but also as an aid to the development of analgesic
procedures for chronic refractory pain.
REFERENCES
1 Adler LJ, Gyulai FE, Diehl DJ, Mintun MA, Winter PM,
Firestone LL. Regional brain activity changes associated with
fentanyl analgesia elucidated by positron emission tomography. Anesth Analg 1997 ; 84 : 120-6.
2 Andersson JL, Lilja A, Hartvig P, Langstrom B, Gordh T,
Handwerker H, et al. Somatotopic organization along the
central sulcus for pain localization in humans, as revealed by
positron emission tomography. Exp Brain Res 1997 ; 117 :
192-9.
3 Apkarian VA, Stea RA, Manglos SH, Szevernyi NM, King RB,
Thomas FD. Persistent pain inhibits contralateral somatosensory cortical activity in humans. Neurosci Lett 1992 ; 140 :
141-7.
4 Aziz Q, Andersson JLR, Valind S, et al. Identification of
human brain loci processing oesophageal sensation using
positron emission tomography. Gastroenterology 1997 ; 113 :
50-9.
5 Baciu MV, Bonaz BL, Papillon E, Bost R, Le Bas JF, Fournet J,
et al. Central processing of rectal pain: a functional MR
imaging study. Am J Neuroradiol 1999 ; 20 : 1920-4.
6 Bancaud J, Talairach J. Clinical semiology of frontal lobe
seizures. Adv Neurol 1992 ; 57 : 3-58.
7 Baron R, Baron Y, Disbrow E, Roberts TPL. Brain processing
of capsaicin-induced secondary hyperalgesia. A functional
MRI study. Neurology 1999 ; 53 : 548-57.
8 Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A,
Comite AR, et al. Human brain activation under controlled
thermal stimulation and habituation to noxious heat: an fMRI
study. Magn Reson in Med 1999 ; 41 : 1044-57.
9 Berthier ML, Starkstein SE, Leiguarda RC. Asymbolia for
pain: a sensory-limbic disconnection syndrome. Ann Neurol
1988 ; 24 : 41-9.
10 Binkofski F, Schnitzler A, Enck P, Frieling T, Posse S, Seitz RJ,
et al. Somatic and limbic cortex activation in esophageal distention: a functional magnetic resonance imaging study. Ann
Neurol 1998 ; 44 : 811-5.
11 Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and
anger. Brain 1999 ; 122 : 883-93.
12 Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional
responses to pleasant and unpleasant music correlate with
activity in para-limbic brain regions. Nature Neurosci 1999 ;
2 : 382-7.
13 Breiter H, Rauch SL, Kwong KK, Baker JR, Weisskoff RM,
Kennedy DN, et al. Functional magnetic resonance imaging of
symptom provocation in obsessive-compulsive disorder. Arch
Gen Psychiatry 1996 ; 53 : 595-606.
14 Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-
284
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
Peyron et al.
hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J Neurosci 1999 ; 25 : 10869-76.
Buckner RL. Event-related fMRI and the hemodynamic response. Hum Brain Map 1998 ; 6 : 373-7.
Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC,
Rauch SL. The counting Stroop: an interference task specialized for functional neuro-imaging. Validation study with functional MRI. Hum Brain Map 1998 ; 6 : 270-82.
Bushnell MC, Duncan GH. Sensory and affective aspects of
pain perception: is medial thalamus restricted to emotional
issues? Exp Brain Res 1989 ; 78 : 415-8.
Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI,
Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci USA 1999 ; 96 : 7705-9.
Cabeza R, Mcintosh AR, Grady CL, Nyberg L, Houle S,
Tulving E. Age-related changes in neural interactions during
memory encoding and retrieval: a network analysis of PET
data. Brain and Cognition 1997 ; 35 : 369-72.
Carter CS, Mintun M, Cohen JD. Interference and facilitation
effects during selective attention: an H215O PET study of
Stroop task performance. Neuroimage 1955 ; 2 : 264-72.
Casey BJ, Cohen JD, Ocraven K, Davidson RJ, Irwin W,
Nelson CA, et al. Reproducibility of fMRI results across four
institutions using a spatial working memory task. Neuroimage
1998 ; 8 : 249-61.
Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ,
Frey KA. Positron emission tomographic analysis of cerebral
structures activated specifically by repetitive noxious heat
stimuli. J Neurophysiol 1994 ; 71 : 802-7.
Casey KL, Minoshima S, Morrow TJ, Koeppe RA, Frey KA.
Imaging the brain in pain: potentials, limitations, and implications. In: Bromm B, Desmedt JE, Eds. Pain and the brain
(Series: Advances in Pain Research and Therapy n° 22). Basel:
Karger; 1995. p. 201-11.
Casey KL, Minoshima S, Morrow TJ, Koeppe RA. Comparison of human cerebral activation patterns during cutaneous
warmth, heat pain and deep cold pain. J Neurophysiol 1996 ;
76 : 571-81.
Chmielowska J, Coghill RC, Maisog JM, Carson RE, Herscovitch P, Honda M, et al. Positron emission tomography [150]
water studies with short interscan interval for single-subject
and group analysis: influence of background subtraction. J
Cereb Blood Flow Metab 1998 ; 18 : 433-43.
Chudler EH, Anton F, Dubner R, Kenshalo DR. Responses of
nociceptive SI neurons in monkeys and pain sensation in
humans elicited by noxious thermal stimulation: effects of
interstimulus interval. J Neurophysiol 1990 ; 63 : 559-69.
Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A,
Bushnell MC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci 1994 ; 14 : 4095-108.
Coghill RC, Sang CN, Berman KF, Bennett GJ, Iadarola MJ.
Global cerebral blood flow decreases during pain. J Cereb
Blood Flow Metab 1998 ; 18 : 141-7.
Coghill RC, Sang CN, Maisog JMA, Iadarola MJ. Pain intensity within the human brain: a bilateral distributed mechanism. J Neurophysiol 1999 ; 82 : 1934-43.
Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color and speed: functional anatomy by
positron emission tomography. J Neurosci 1991 ; 11 : 2383402.
Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET
study of visuospatial attention. J Neurosci 1993 ; 13 : 1202-26.
Coull JT, Nobre AC. Where and when to pay attention: the
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
neural systems for directing attention to spatial locations and
to time intervals as revealed by both PET and fMRI. J Neurosci
1998 ; 18 : 7426-35.
Craig AD. Supraspinal projections of lamina I neurons. In:
Besson JM, Guilbaud G, Ollat H, Eds. Forebrain areas involved in pain processing. Paris: John Libbey; 1995. p. 13-26.
Craig AD, Reiman EM, Evans A, Bushnell MC. Functional
imaging of an illusion of pain. Nature 1996 ; 384 : 258-60.
Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory
activation of insular cortex. Nat Neurosci 2000 ; 3 : 184-90.
Créac’h C, Henry P, Caille JM, Allard M. Functional MRI
analysis of pain-related brain activation following acute mechanical stimulation. Am J Neuroradiology 2000 ; 21 (in
press).
Davis KD, Wood ML, Crawley AP, Mikulis DJ. fMRI of
human somatosensory and cingulate cortex during painful
electrical nerve stimulation. NeuroReport 1995 ; 7 : 321-5.
Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ.
Functional MRI of pain and attention-related activations in
the human cingulate cortex. J Neurophysiol 1997 ; 77 :
3370-80.
Davis KD, Kwan CL, Crawley AP, Mikulis DJ. Functional
MRI study of thalamic and cortical activations evoked by
cutaneous heat, cold, and tactile stimuli. J Neurophysiol
1998 ; 80 : 1533-46.
Davis KD, Lozano RM, Manduch M, Tasker RR, Kiss ZH,
Dostrovsky JO. Thalamic relay site for cold perception in
humans. J Neurophysiol 1999 ; 81 : 1970-3.
Derbyshire SWG, Jones AKP. Cerebral responses to a continual tonic pain stimulus measured using positron emission
tomography. Pain 1998 ; 76 : 127-35.
Derbyshire SWG, Jones AKP, Devani P, Friston KJ, Feinmann C, Harris M, et al. Cerebral responses to pain in patients
with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg Psychiatry 1994 ; 57 : 116672.
Derbyshire SWG, Jones AKP, Gyulai F, Clarck S,
Townsend D, Firestone LL. Pain processing during three levels
of noxious stimulation produces differential patterns of central
activity. Pain 1997 ; 73 : 431-45.
Derbyshire SWG, Vogt BA, Jones AKP. Pain and Stroop
interference tasks activate separate processing modules in anterior cingulate cortex. Exp Brain Res 1998 ; 118 : 52-60.
Derbyshire SW, Jones AK, Collins M, Feinmann C, Harris M.
Cerebral responses to pain in patients suffering acute postdental extraction pain measured by positron emission tomography (PET). Eur J Pain 1999 ; 3 : 103-13.
Desmedt JE, Huy NT, Bourget M. The cognitive P40, N60
and P100 components of somatosensory evoked potentials and
the earliest electrical signs of sensory processing in man. Electroencephal Clin Neurophysiol 1983 ; 56 : 272-82.
Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior
cingulate cortex to behaviour. Brain 1995 ; 118 : 279-306.
Di Piero V, Jones AK, Iannotti F, Powell M, Perani D,
Lenzi GL, et al. Chronic pain: a PET study of the central effects
of percutaneous high cervical cordotomy. Pain 1991 ; 46 :
9-12.
Di Piero V, Fiacco F, Tombari D, Pantano P. Tonic pain: a
SPET study in normal subjects and cluster headache patients.
Pain 1997 ; 70 : 185-91.
Dong WK, Ryu H, Wagman IH. Nociceptive responses of
neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol 1978 ; 4 : 1592-613.
Dostrovsky JO, Hutchison WD, Davis KD, Lozano A. Potential role of orbital and cingulate cortices in nociception. In:
Brain responses to pain
52
53
54
55
56
57
58
59
60
61
62
63
64
65
66
67
68
69
Besson JM, Guilbaud G, Ollat H, Eds. Forebrain areas involved in pain processing. Paris: John Libbey Eurotext; 1995.
p. 171-81.
Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR,
Raichle ME. Blood flow changes in human somatosensory
cortex during anticipated stimulation. Nature 1995 ; 373 :
249-52.
Drevets WC, Price JL, Simpson JR, Todd RD, Reich T,
Vannier M, et al. Subgenual prefrontal cortex abnormalities in
mood disorders. Nature 1997 ; 386 : 824-7.
Duncan G, Kupers RC, Marchand S, Villemure JG, Gybels JM, Bushnell MC. Stimulation of human thalamus for
pain relief: possible modulatory circuits revealed by Positron
Emission Tomography. J Neurophysiol 1998 ; 80 : 3326-30.
Farah MJ, Aguirre GK. Imaging visual recognition: PET and
fMRI studies of the functional anatomy of human visual
recognition. Trends in Cognitive Sciences 1999 ; 3 : 179-86.
Faurion A, Cerf B, Van De Moortele PF, Lobel E, MacLeod P,
Le Bihan D. Human taste cortical areas studied with functional magnetic resonance imaging: evidence of functional
lateralization related to handedness. Neurosci Lett 1999 ; 31 :
189-92.
Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD.
Space-based and object-based visual attention: shared and
specific neural domains. Brain 1997 ; 120 : 2013-28.
Firestone LL, Gyulai F, Mintun M, Adler LJ, Urso K, Winter PM. Human brain activity response to fentanyl imaged by
positron emission tomography. Anesth Analg 1996 ; 82 :
1247-51.
Fitzgerald PJ, Lane JW, Hsiao SS. Attentional effects in somatosensory cortex during an orientation discrimination task.
Proc Soc Neurosci [abstract] 1998 ; 24 : 44410.
Folz EL, White LE. Pain ‘relief’ by frontal cingulumotomy. J
Neurosurg 1962 ; 19 : 89-100.
Frederikson M, Wik G, Fischer H, Andersson J. Affective and
attentive neural networks in humans: a PET study of Pavlovian
conditioning. NeuroReport 1995 ; 7 : 97-101.
Frot M, Mauguière F. Timing and spatial distribution of
somatosensory responses recorded in the upper bank of the
sylvian fissure (SII area) in humans. Cereb Cortex 1999 ; 9 :
854-63.
Frot M, Rambaud L, Guénot M, Mauguière F. Intracortical
recordings of early pain-related CO2-laser potentials in the
human second somatosensory (SII) area. Clin Neurophysiol
1999 ; 110 : 133-45.
Frot M, Isnard J, Guénot M, Mauguière F. Effects of noxious
stimulus intensity on signals from operculo-insular cortex: an
intra-cerebral recordings study in humans. Clin Neurophysiol
2000 ; 111 Suppl 1 : 131.
García-Larrea L, Bastuji H, Mauguière F. Mapping study of
selective attentional effects on somatosensory evoked potentials. Electroencephal Clin Neurophysiol 1991 ; 82 : 101-14.
García-Larrea L, Peyron R, Laurent B, Mauguière F. Association and dissociation between laser-evoked potentials and pain
perception. NeuroReport 1997 ; 8 : 3785-9.
García-Larrea L, Peyron R, Mertens P, Grégoire MC,
Costes N, Lavenne F, et al. Electrical stimulation of motor
cortex for pain control: a combined PET-scan and electrophysiological study. Pain 1999 ; 83 : 259-73.
George MSES, Ketter TA, Parekh PI, Rosinsky N, Ring H,
Casey BJ, et al. Regional brain activity when selecting a response despite interference An H2O15 PET study of the
Stroop and an emotional Stroop. Hum Brain Map 1994 ; 1 :
194-209.
Ginsberg MD, Yoshii F, Vilbulsresth D, Chang JY, Duara R,
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
285
Barker WW, et al. Human task-specific somatosensory activation. Neurology 1987 ; 37 : 1301-8.
Gitelman DR, Alpert NM, Kosslyn S, Daffner K, Scinto L, et
al. Functional Imaging of human right hemispheric activation
for exploratory movements. Ann Neurol 1996 ; 39 : 174-9.
Grafton ST, Woods RP, Tyszka M. Functional imaging of
procedural motor learning: relating cerebral blood flow with
individual subject performance. Hum Brain Map 1994 ; 1 :
221-34.
Greenspan JD, Thomadaki M, Mcgillis SLB. Spatial summation of perceived pressure, sharpness and mechanically evoked
cutaneous pain. Somatosens Mot Res 1997 ; 14 : 107-12.
Hautvast RWM, Terhorst GJ, Dejong BM, Dejongste MJL,
Blanksma PK, Paans AMJ, et al. Relative changes in regional
cerebral blood flow during spinal cord stimulation in patients
with refractory angina pectoris. Eur J Neurosci 1997 ; 9 :
1178-83.
Hsieh JC, Hagermark O, Stahle-Backdahl M, Ericson K,
Eriksson L, Stone-Elander S, et al. Urge to scratch represented
in the human cerebral cortex during itch. J Neurophysiol
1994 ; 72 : 3004-8.
Hsieh JC, Bäckdahl MS, Hägermark Ö, Stone-Elander S,
Rosenquist G, Ingvar M. Traumatic nociceptive pain activates
the hypothalamus and the periaqueductal gray: a positron
emission tomography study. Pain 1995 ; 64 : 303-14.
Hsieh JC, Belfrage M, Stone-Elander S, Hansson P, Ingvar M.
Central representation of chronic ongoing neuropathic pain
studied by positron emission tomography. Pain 1995 ; 63 :
225-36.
Hsieh JC, Hannerz J, Ingvar M. Right-lateralized central processing for pain of nitroglycerin-induced cluster headache.
Pain 1996 ; 67 : 59-68.
Hsieh JC, Stone-Elander S, Ingvar M. Anticipatory coping of
pain expressed in the human anterior cingulate cortex: a
positron emission tomography study. Neurosci Lett 1999 ;
262 : 61-4.
Hutchison WD, Davis KD, Lozano AM, Tasker RR, Dostrovsky JO. Pain-related neurons in the human cingulate cortex.
Nat Neurosci 1999 ; 2 : 403-5.
Iadarola MJ, Max MB, Berman KF, Byas-Smith MG,
Coghill RC, Gracely RH, Bennett GJ. Unilateral decrease in
thalamic activity observed with positron emission tomography
in patients with chronic neuropathic pain. Pain 1995 ; 63 :
55-64.
Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG,
Gracely RH, Max MB, et al. Neural activation during acute
capsaicin-evoked pain and allodynia assessed with PET. Brain
1998 ; 121 : 931-47.
Iriki A, Tanaka M, Iwamura Y. Attention-induced neuronal
activity in the monkey somatosensory cortex revealed by pupillometrics. NeurosciRes 1996 ; 25 : 173-81.
Jones AKP, Derbyshire SWG. Cortical and thalamic imaging
in normal volunteers and patients with chronic pain. In:
Besson JM, Guilbaud G, Ollat H, Eds. Forebrain areas involved in pain processing. Paris: John Libbey Eurotext; 1995.
p. 229-38.
Jones AKP, Derbyshire SW. Reduced cortical responses to
noxious heat in patients with rheumatoid arthritis. Ann
Rheum Dis 1997 ; 56 : 601-7.
Jones AKP, Brown WD, Friston KJ, Qi LY, Frackowiak RSJ.
Cortical and subcortical localization of response to pain in man
using positron emission tomography. Proc R Soc Lond B
1991 ; 244 : 39-44.
Jones AKP, Qi LY, Fujirawa T, Luthra SK, Ashburner J,
Bloomfield P, et al. In vivo distribution of opioid receptors in
286
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
Peyron et al.
man in relation to the cortical projections of the medial and
lateral pain systems measured with positron emission tomography. Neurosci Lett 1991 ; 126 : 25-8.
Jones AKP, Cunningham VJ, Ha-Kawa S, et al. Changes in
central opioids receptor binding in relation to inflammation
and pain in patients with rheumatoid arthritis. Br J Rheumatol
1994 ; 33 : 909-16.
Jueptner M, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE. Anatomy of motor learning. II. Subcortical structures
and learning by trial and error. J Neurophysiol 1997 ; 77 :
1325-37.
Karbe H, Herholz K, Weberluxenburger G, Ghaemi M,
Heiss WD. Cerebral networks and functional brain asymmetry: evidence from regional metabolic changes during word
repetition. Brain and Language 1998 ; 63 : 108-21.
Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R,
Koyama M, et al. Functional anatomy of GO/NO-GO discrimination and response selection. A PET study in man.
Brain Res 1996 ; 728 : 79-89.
Kenshalo DR Jr, Chudler EH, Anton F, Dubner R. SI nociceptive neurons participate in the encoding process by which
monkeys perceive the intensity of noxious thermal stimulation. Brain Res 1988 ; 454 : 378-82.
Klingberg T. Concurrent performance of two working
memory tasks: potential mechanisms of interference. Cereb
Cortex 1998 ; 8 : 593-601.
Koyama T, Tanaka YZ, Mikami A. Nociceptive neurons in the
macaque anterior cingulate activate during anticipation of
pain. NeuroReport 1998 ; 9 : 2663-7.
Kupers RC, Jensen TS, Gybels JM, Giedde A. PET activation
study in a patient with neuropathic pain is successfully treated
with thalamic stimulation. Soc Neurosci (LA) 1998 ; 547 :
1387.
Lewin JS, Friedman L, Wu D, Miller DA, Thompson LA,
Klein SK, et al. Cortical localization of human sustained
attention: detection with functional MR using a visual vigilance paradigm. J Comput Assist Tomogr 1996 ; 20 : 695-701.
Madden DJ, Turkington TG, Provenzale JM, Hawk TC,
Hoffman JM, Coleman RE. Selective and divided visual attention: age-related changes in regional cerebral blood flow measured by H215O PET. Hum Brain Map 1997 ; 5 : 389-409.
Mauguière F, Merlet I, Forss N, Vanni S, Jousmäki V,
Adeleine P, et al. Activation of a distributed somatosensory
cortical network in the human brain. A dipole modelling study
of magnetic fields evoked by median nerve stimulation. Part I:
Location and activation timing of SEF sources. Electroencephalogr Clin Neurophysiol 1997 ; 104 : 281-9.
May A, Bahra A, Büchel C, Frackowiak RSJ, Goadsby PJ.
Hypothalamic activation in cluster headache attacks. Lancet
1998 ; 352 : 275-8.
May A, Kaube H, Büchel C, Eichten C, Rijntjes M, Jüptner M, et al. Experimental pain elicited by capsaicin: a PET
study. Pain 1998 ; 74 : 61-6.
McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent
events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol 1997 ; 77 :
1630-4.
Melzack R, Casey KL. Sensory, motivational, and central
control determinants of pain. In: Kenshalo DR, Ed. The skin
senses. Springfield, IL: CC Thomas; 1968. p. 423-39.
Melzack R, Katz J. Pain measurements in persons in pain. In:
Wall PD, Melzack R, Eds. Textbook of pain. Edinburgh:
Churchill Livingstone; 1994. p. 337-51.
Menon V, Ford JM, Lim KO, Glover GH, Pfefferbaum A.
Combined event-related fMRI and EEG evidence for
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
119
120
temporal-parietal cortex activation during target detection.
NeuroReport 1997 ; 8 : 3029-37.
Mesulam MM. From sensation to cognition. Brain 1998 ;
121 : 1013-52.
Meyer E, Ferguson SG, Zarorre RJ, Alivisaros B, Marrett S,
Evans AC, et al. Attention modulates somatosensory cerebral
blood flow response to vibrotactile stimulation as measured by
positron emission tomography. Ann Neurol 1991 ; 29 : 440-3.
Mima T, Nagamine T, Nakamura K, Shibasaki H. Attention
modulates both primary and second somatosensory cortical
activities in humans: a magnetoencephalographic study. J
Neurophysiol 1998 ; 80 : 2215-21.
Miron D, Duncan GH, Bushnell MC. Effects of attention on
the intensity and unpleasantness of thermal pain. Pain 1989 ;
39 : 345-52.
Morris JS, Friston KJ, Büchel C, Frith CD, Young AW,
Calder AJ, et al. A neuromodulatory role for the human
amygdala in processing emotional facial expressions. Brain
1998 ; 121 : 47-57.
Morris JS, Scott SK, Dolan RJ. Saying it with feeling: neural
responses to emotional vocalizations. Neuropsychologia
1999 ; 37 : 1155-63.
Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A.
Anticipation causes increased blood flow to the anterior cingulate cortex. Hum Brain Map 1996 ; 4 : 103-12.
Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM,
Frackowiak RSJ, Frith CD. Functional localization of the
system for visuospatial attention using positron emission tomography. Brain 1997 ; 120 : 515-33.
O’Leary DS, Andreasen NC, Hurtig RR, Torres IJ, Flashman LA, Kesler ML, et al. Auditory and visual attention
assessed with PET. Hum Brain Map 1997 ; 5 : 422-36.
Opsommer E, Masquelier E, Plaghki L. Determination of
nerve conduction velocity of C-fibres in humans from thermal
thresholds to contact heat (thermode) and from evoked brain
potentials to radiant heat (CO2 laser). Neurophysiol Clin
1999 ; 29 : 411-22.
Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior
cingulate cortex mediates processing selection in the Stroop
attentional conflict paradigm. Proc Natl Acad Sci USA 1990 ;
87 : 256-9.
Pardo JV, Fox PT, Raichle ME. Localization of a human
system for sustained attention by positron emission tomography. Nature 1991 ; 349 : 61-4.
Paulson PE, Minoshima S, Morrow TJ, Casey KL. Gender
differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 1998 ;
76 : 223-9.
Penfield W, Boldrey EB. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical
stimulation. Brain 1937 ; 60 : 389-443.
Petrovic P, Ingvar M, Stone-Elander S, Petersson KM, Hansson P. A PET activation study of dynamic mechanical allodynia in patients with mononeuropathy. Pain 1999 ; 83 :
459-70.
Peyron R, García-Larrea L, Deiber MP, Cinotti L, Convers P,
Sindou M, et al. Electrical stimulation of precentral cortical
area in the treatment of central pain. Electrophysiological and
PET study. Pain 1995 ; 62 : 275-86.
Peyron R, Garcia-Larrea L, Grégoire MC, Convers P, Cinotti
L, Michel D, et al. Positron Emission Tomography (PET)
evidence of Cerebral Blood Flow (CBF) abnormalities in patients with neurological pain after lateral-medullary infarct
(Wallenberg’s syndrome WS). VIIIth World Congress On
Pain, IASP, August 1996, Vancouver [abstract].
Brain responses to pain
121 Peyron R, García-Larrea L, Grégoire MC, Convers P,
Lavenne F, Veyre L, et al. Allodynia after lateral-medullary
(Wallenberg) infarct. A Positron Emission Tomography
(PET) study. Brain 1998 ; 121 : 345-56.
122 Peyron R, García-Larrea L, Grégoire MC, Costes N, Convers P, Lavenne F, et al. Haemodynamic brain responses to
acute pain in humans: sensory and attentional networks. Brain
1999 ; 122 : 1765-79.
123 Peyron R, García-Larrea L, Mertens P, Costes N, Mauguière
F, Michel D, et al. Temporal pattern of cerebral blood flow
(CBF) in patients with electrical motor cortex stimulation
(MCS) for pain control. Soc Neurosci (Miami) October 1999
[abstract].
124 Peyron R, García-Larrea L, Grégoire MC, Convers P, Richard A, Manet L, et al. Parietal and cingulate processings in
central pain. A positron emission tomography (PET) study of
one original case. Pain 2000 ; 84 : 77-87.
125 Phillips ML, Young AW, Senior C, Brammer M, Andrew C,
Calser AJ, et al. A specific neural substrate for perceiving facial
expressions of disgust. Nature 1997 ; 389 : 495-8.
126 Picard N, Strick PL. Motor areas of the medial wall: a review of
their location and functional activation. Cereb Cortex 1996 ;
6 : 342-53.
127 Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, et al. Dissociating pain from its anticipation in the
human brain. Science 1999 ; 284 : 1979-81.
128 Porro CA, Cettolo V, Francescato MP, Baraldi P. Temporal
and intensity coding of pain in human cortex. J Neurophysiol
1998 ; 80 : 3312-20.
129 Portas CM, Rees G, Howseman AM, Josephs O, Turner R,
Frith CD. A specific role for the thalamus in mediating the
interaction of attention and arousal in humans. J Neurosci
1998 ; 18 : 8979-89.
130 Posner MI. Attention: the mechanisms of consciousness. Proc
Natl Acad Sci USA 1994 ; 91 : 7398-403.
131 Posner MI, Dehaene S. Attentional networks. Trends Neurosci 1994 ; 17 : 75-9.
132 Price DD. The question of how the dorsal horn encodes
sensory information. In: Yaksh TL, Ed. Spinal afferent processing. New York: Plenum Press; 1986. p. 445-66.
133 Raichle ME, Fiez JA, Videen TO, MacLeod AMK, Pardo JV,
Fox P, et al. Practice-related changes in human brain functional anatomy during non-motor learning. Cereb Cortex
1994 ; 4 : 8-26.
134 Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC.
Pain affect encoded in human anterior cingulate but not
somatosensory cortex. Science 1997 ; 277 : 968-71.
135 Rainville P, Hofbauer RK, Paus T, Duncan GH, Bushnell MC, Price DD. Cerebral mechanisms of hypnotic induction and suggestion. J Cognitive Neurosci 1999 ; 11 : 110-25.
136 Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter H, Savage CR, et al. Regional cerebral blood flow measured during
symptom provocation in obsessive-compulsive disorder using
oxygen 15-labeled carbon dioxide and Positron Emission Tomography. Arch Gen Psychiatry 1994 ; 51 : 62-70.
137 Rauch SL, Savage CR, Alpert NM, Miguel EC, Baer L,
Breiter H, et al. A Positron Emission Tomographic study of
simple phobic symptom provocation. Arch Gen Psychiatry
1995 ; 52 : 20-8.
138 Rauch SL, Van der Kolk BA, Fisler RE, Alpert NM, Orr SP,
Savage CR, et al. A symptom provocation study of posttraumatic stress disorder using Positron Emission Tomography and script-driven imagery. Arch Gen Psychiatry 1996 ;
53 : 380-7.
139 Rees G, Howseman A, Josephs O, Frith CD, Friston KJ,
140
141
142
143
144
145
146
147
148
149
150
151
152
153
154
155
156
287
Frackowiak RS, et al. Characterizing the relationship between
BOLD contrast and regional cerebral blood flow measurements by varying the stimulus presentation rate. Neuroimage
1997 ; 6 : 270-8.
Rosen BR, Buckner RL, Dale AM. Event-related functional
MRI: past, present, and future. Proc Natl Acad Sci USA 1998 ;
95 : 773-80.
Rosen SD, Paulesu E, Frith CD, Frackowiak RSJ, Davies GJ,
Jones T, et al. Central nervous pathways mediating angina
pectoris. Lancet 1994 ; 344 : 147-50.
Sadato N, Yonekura Y, Yamada H, Nakamura S, Waki A,
Ishii Y. Activation patterns of covert word generation detected
by fMRI: comparison with 3D PET. J Comput Assist Tomogr
1998 ; 22 : 945-52.
Siedenberg R, Treede RD. Laser-evoked potentials: exogenous
and endogenous components. Electroencephal Clin Neurophysiol 1996 ; 100 : 240-9.
Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit
cingulate cortex. J Neurophysiol 1992 ; 68 : 1720-32.
Silverman DHS, Munakata JA, Ennes H, et al. Regional
cerebral activity in normal and pathological perception of
visceral pain. Gastroenterology 1997 ; 112 : 64-72.
Small DM, Zald DH, Jones-Gotman M, Zatorre RJ,
Pardo JV, Frey S, et al. Human cortical gustatory areas: a
review of functional neuroimaging data. NeuroReport 1999 ;
18 : 7-14.
Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, et al. The [14C]deoxyglucose method
for the measurement of local cerebral glucose utilization:
theory, procedure and normal values in the conscious and
anesthetized albino rat. J Neurochem 1977 ; 28 : 897-916.
Sokoloff L, Porter A, Roland P, Wise O, Frakowiack RH,
Jones T, et al. General discussion. In: Chadwick C, Derek J,
Whelan J, Eds. Exploring brain functional anatomy with
positron emission tomography. Ciba Foundation Symposium
n° 163. London: Wiley & Sons; 1991. p. 43-56.
Stohler CS, Kowalski CJ. Spatial and temporal summation of
sensory and affective dimensions of deep somatic pain. Pain
1999 ; 79 : 165-73.
Svensson P, Minoshima S, Beydoun A, Morrow TJ, Casey KL.
Cerebral processing of acute skin and muscle pain in humans.
J Neurophysiol 1997 ; 78 : 450-60.
Svensson P, Johannsen P, Jensen TS, Arend-Nielsen L,
Nielsen J, Stodkilde-Jorgersen H, et al. A cerebral representation of graded painful phasic and tonic heat in humans: a
positron emission tomography stimuli. In: Jensen TS,
Turner JA, Wiesenfeld-Hallin Z, Eds. Proceedings of the 8th
World Congress on Pain. Progress in pain research and management, Vol. 8. Seattle: IASP Press; 1997. p. 867-78.
Talairach J, Tournoux P. Coplanar stereotaxic atlas of the
human brain. Stuttgart: Thieme; 1988.
Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC,
Duncan GH. Multiple representations of pain in human cerebral cortex. Science 1991 ; 251 : 1355-8.
Talbot JD, Villemure JG, Bushnell MC, Duncan GH. Evaluation of pain perception after anterior capsulotomy: a case
report. Somatosens and Mot Res 1995 ; 12 : 115-26.
Taylor SF, Kornblum S, Minoshima S, Oliver LM,
Koeppe RA. Changes in medial cortical blood flow with a
stimulus-response compatibility task. Neuropsychologia
1994 ; 32 : 249-55.
Tölle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, et al. Region-specific encoding of sensory and
affective components of pain in the human brain: a Positron
288
157
158
159
160
161
162
Peyron et al.
Emission Tomography correlation analysis. Ann Neurol
1999 ; 45 : 40-7.
Turken AU, Swick D. Response selection in the human anterior cingulate cortex. Nat Neurosci 1999 ; 2 : 920-4.
Turner R. Magnetic resonance imaging of brain function. Am
J Physiol Imaging 1992 ; 3/4 : 136-45.
Vaccarino A, Melzack R. Analgesia produced by injection of
lidocaine into the anterior cingulum bundle of the rat. Pain
1989 ; 39 : 213-9.
Valeriani M, Restuccia D, Di Lazzaro V, Oliviero A, Profice P,
Le Pera D, et al. Inhibition of the human primary motor area
by painful heat stimulation of the skin. Clin Neurophysiol
1999 ; 110 : 1475-80.
Vogt BA, Watanabe H, Grootoonk S, Jones AKP. Topography of diprenorphine binding in human cingulate gyrus and
adjacent cortex derived from coregistered PET and MR images. Hum Brain Map 1995 ; 3 : 1-12.
Vogt BA, Derbyshire S, Jones AKP. Pain processing in four
regions of human cingulate cortex localized with co-registered
PET and MR imaging. Eur J Neurosci 1996 ; 8 : 1461-73.
163 Whalen PJ, Bush G, McNally R, Wilhelm S, McInerney SC,
Jenike MA, et al. The emotional counting Stroop paradigm: a
functional Magnetic Resonance Imaging probe of the anterior
cingulate affective division. Biol Psychiatry 1998 ; 44 : 121928.
164 Wildgruber D, Kischka U, Ackermann H, Klose U, Grodd W.
Dynamic pattern of brain activation during sequencing of
word strings evaluated by fMRI. Cognitive Brain Res 1999 ; 7 :
285-94.
165 Xu X, Fukuyama H, Yazawa S, Mima T, Hanakawa T, Magata Y, et al. Functional localization of pain perception in the
human brain studied by PET. NeuroReport 1997 ; 8 : 555-9.
166 Zarahn E, Aguirre GK, DEsposito M. Temporal isolation of
the neural correlates of spatial mnemonic processing with
fMRI. Cogn Brain Res 1999 ; 7 : 255-68.
167 Zhang ZH, Dougherty PM, Oppenheimer SM. Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience 1999 ; 94 : 351-60.
168 Zhou YD, Fuster JM. Mnemonic neuronal activity in somatosensory cortex. Proc Natl Acad Sci USA 1996 ; 93 : 10533-7.