Shelf stability of lutein from marigold (Tagetes erecta L
advertisement

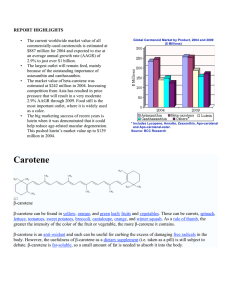
Shelf stability of lutein from marigold (Tagetes erecta L.) flowers in vegetable oils Roberto Lavecchia and Antonio Zuorro Department of Chemical Engineering, “Sapienza” University Via Eudossiana, 18 – 00184 Roma (Italy) The stability of lutein in sunflower and rice bran oils was investigated in the temperature range 25–40 °C. Experiments were made using a standardized plant extract at 5% by weight of lutein derived from the flowers of Tagetes erecta L. Samples of the pigment in oil were incubated at each temperature for up to 10 days and the time course of degradation was monitored. A kinetic analysis of the data showed that thermal degradation follows first-order kinetics, with apparent activation energies of 60.9 kJ mol−1 (in sunflower oil) and 44.9 kJ mol−1 (in rice bran oil). From the estimated kinetic parameters, the carotenoid half-life at 4 and 20 °C were determined. The results obtained indicate that lutein is more stable in sunflower oil. The observed differences in stability could be a reflection of the different fatty acid composition of the two oils and the presence of endogenous antioxidants. 1. Introduction Lutein is a carotenoid pigment found in high concentrations in cruciferous and green leafy vegetables (Landrum et al., 2002). It is also present in significant amounts in maize, alfalfa and the petals of marigold (Tagetes erecta L.), from which it is extracted for commercial use. Chemically, lutein (5¶50FDURWHQH¶GLRO is a dihydroxy derivative RI.FDURWHQHZLWKK\GUR[\O groups located on the 3 and 3’ carbons (Figure 1). Of the many carotenoids circulating in human plasma, only lutein and its structural isomer zeaxanthin are accumulated throughout the tissues of the eye (Bone et al., 1997). In the macular region of the retina they can reach concentrations as high as 1 pmol per square millimeter of tissue. Increasing evidence from epidemiological and experimental studies suggests that these macular carotenoids might have a role in maintaining or improving vision and, more importantly, in preventing onset or progression of agerelated macular degeneration (Snodderly, 1995). The mechanism by which they exert their beneficial effects needs to be further elucidated but is probably twofold (Kirschfeld, 1982; Palozza and Krinsky, 1992). First, these carotenoids absorb light in the blue range, which is thought to be very harmful to the retina, thereby reducing the associated photo-oxidative damage. A second potential function involves their antioxidant properties, i.e., the ability to quench free radicals and other reactive oxygen species, whose concentrations are particularly high in the retinal tissue. As diet is the only source of lutein and zeaxanthin, enhancement of their intake by dietary supplementation or by fortification of foods is being actively considered as a means to increase their levels of in the organism. Figure 1 – Molecular structure of lutein. In the past few years we have successfully included lutein, alone or in combination with other carotenoids, in yogurts and other food products. However, to ensure product quality and food safety an accurate prediction of shelf-life is also required. In this paper we present the results of an experimental study on the stability of lutein in sunflower and rice bran oils. Analysis of degradation rate data indicated that lutein is more stable in sunflower than in rice bran oil. The kinetic parameters estimated from these data allowed prediction of shelf-life for the two systems under different storage conditions. 2. Experimental 2.1 Materials A standardized dry powder extract from the flowers of Tagetes erecta L. (at 5% by weight of lutein) was obtained from SCIDOOR HI−TECH BIOLOGY CO. (Shaanxi, China). Sunflower and rice bran oils were purchased from a local market and stored at room temperature in the dark. 2.2 Methods Solutions of different lutein concentrations were prepared by dissolving, under stirring, appropriate amounts of the powdered extract in the vegetable oils. Samples of the products were incubated at the desired temperature (25 or 40 °C) in a refrigerated incubator (FTC 90E, Velp Scientifica, Italy) for up to 10 days. At selected times, aliquots were withdrawn and analysed for lutein content. Lutein concentrations were determined by spectrophotometric measurement at 452 nm, where the absorption spectrum of the pigment displays a sharp peak. A double-beam UV–VIS spectrophotometer (Perkin–Elmer Lambda 25) and quartz cells of 1-cm path length were used. 3. Results and Discussion Typical results showing the time variation of absorbance at 452 nm are presented in Figure 2. The observed absorbance changes were largely irreversible and were attributed to thermal degradation of lutein. Degradation rates were calculated from the slope of the absorbance−time plots. At each A452 1.2 T = 40 °C 1 0.8 0.6 0.4 0.2 0 40 80 120 160 200 t, h Figure 2 – Time changes of absorbance at 452 nm for lutein in sunflower oil at different initial carotenoid concentrations. temperature and in each vegetable oil they were found to be linearly related to the undegraded lutein concentration (Figure 3). Accordingly, the kinetic data were interpreted by a first-order rate expression: (1) r = k(T) c where r is the degradation rate, k is the first-order rate constant and c is the lutein concentration. To describe the temperature dependence of k we assumed the Arrhenius equation: E k ( T ) = k0 exp − a RT (2) 12 a 8 r 104, h -1 r 104, h -1 10 40 °C 6 40 °C 6 25 °C 4 b 9 25 °C 3 2 0 0 0 0.3 0.6 0.9 1.2 1.5 A 0 0.2 0.4 0.6 0.8 1 1.2 A Figure 3 – Kinetic plots showing the dependence of degradation rate (r) on absorbance at 452 nm in: (a) sunflower oil and (b) rice bran oil. where Ea is the apparent activation energy for degradation. Rate constants and activation energies were determined by least squares regression, leading to the results summarized in Table 1. In both oils, as expected, lutein degradation increased with temperature. Values of the first-order rate constant were higher in rice bran oil than in sunflower oil, suggesting that lutein was more stable in the latter. According to the apparent activation energies, stability in sunflower oil (Ea = 60.9 kJ/mol) is more sensitive to temperature than in rice bran oil (Ea = 44.9 kJ/mol). These results cannot be directly compared with literature values due to the lack of kinetic studies on the degradation of this carotenoid. Apparent activation energies, however, are close to the value of 59.6 kJ/mol found for lycopene degradation in sunflower oil (Lavecchia and Zuorro, 2006). An activation energy of 61 kJ/mol is also reported by Lee and Chen (2002) for the degradation of a thin vacuumdeposited film of lycopene. Recently Koca et al. (2007) investigated the kinetics of colour changes in dehydrated blanched and unblanched carrots during storage. The DXWKRUV IRXQG D ILUVWRUGHU NLQHWLFV IRU WKHUPDO GHJUDGDWLRQ RI FDURWHQH ZLWK activation energies of 66.1 kJ/mol and 38.9 kJ/mol, respectively, for blanched and unblanched samples. In contrast, higher values (between 82.8 and 109.6 kJ/mol) were obtained for the oxidative degradation of carotenoids, including lutein, in safflower oil (Henry et al., 1998). This study, however, was conducted at considerably higher temperatures (75–95 °C) and it cannot be excluded that, under these conditions, degradation might follow different pathways. To quantify the storage stability of lutein-based products we determined their apparent half-life, i.e., the time required for half of the initial amount of the pigment to disappear. For first-order kinetics, half-life depends only on temperature and can be calculated as: τ= ln 2 k( T ) (3) Half-lives in sunflower oil were equal to 101.2 days, at 25 °C, and 31.4 days at 40 °C. In rice bran oil the corresponding values were 62.3 and 26.3 days, respectively. Figure 4 shows the effect of temperature on the half-life of lutein in the two vegetable oils. As is evident, the stability curve in sunflower oil is constantly over that in rice bran oil. Differences, however, decrease with temperature and tend to disappear above 45–50 °C. Table 1 – Kinetic parameters for lutein degradation in the two vegetable oils. Vegetable oil Sunflower Rice bran T (°C) 25 40 25 40 k⋅104 (h−1) 2.33 ± 0.48 7.56 ± 0.66 4.61 ± 0.32 11.10 ± 0.74 Ea (kJ mol−1) 60.9 ± 3.2 44.9 ± 2.7 τ, days 1000 T = 4 °C 800 600 a 400 T = 20 °C b 200 0 0 5 10 15 20 25 30 35 40 T, °C Figure 4 – Effect of temperature on the half-life of lutein (τ) in: (a) sunflower oil and (b) rice bran oil. The reason for the observed differences in stability is to be found in the different properties of the two oils. As is illustrated by the values in Table 2, they differ both in fatty acid composition and in the type and level of antioxidant compounds. It is generally accepted that the higher the degree of unsaturation of a vegetable oil, the faster is oxidative deterioration (Chan, 1987; Porter et al., 1995). Oxidation leads to the formation of highly reactive species, such as alkyl and peroxyl radicals, which can, in turn, increase the degradation of easily oxidizable compounds, as is lutein in our case. On the basis of these considerations, lutein should be more stable in rice bran oil, due to its lowest degree of unsaturation. Stability, however, is also affected by the presence of endogenous antioxidants, such as tocopherols and tocotrienols. Studies performed in non-polar environments have shown that tocopherols can protect β-carotene from oxidative damage (Mortensen and Skibsted, 1997a; Krinsky and Yeum, 2003). Similar results were obtained for lycopene (Mortensen and Skibsted, 1997b). Table 2 – Fatty acid composition and antioxidant content of sunflower and rice bran oils (SFA: saturated fatty acids; MUFA: mono-unsaturated fatty acids; PUFA: polyunsaturated fatty acids). Source: Codex Alimentarius Commission, 2001. Vegetable oil Sunflower Rice bran SFA (wt %) 12 20 MUFA (wt %) 21 45 PUFA (wt %) 67 35 Tocopherols (mg/100 g) 40.3–102.0 25.6−64.8 Tocotrienols (mg/100 g) − 24.4−104.1 The underlying hypothesis is that tocopherols can regenerate the biologically active carotenoid molecules by an electron transfer mechanism (Mortensen et al., 2001). As regards the levels of endogenous antioxidants in the two oils, we note from the values given in Table 2 that sunflower oil contains more tocopherols than does rice bran oil, even if the latter has some additional amount of tocotrienols. We can, therefore, hypothesize that at lower temperatures lutein degrades slowly in sunflower oil because of the higher content of tocopherols. As the temperature is increased, the protection offered by tocopherols may be overcome by the formation of reactive species caused by lipid peroxidation. Due to these opposing effects, differences in stability in the two vegetable oils tend to vanish, making it almost independent of oil type at temperatures higher than 45–50 °C. In conclusion, it may be interesting to estimate lutein stability at two significant storage temperatures: 4 and 20 °C. By the use of eqn. (3) and the kinetic parameters obtained previously, at 4 °C we determined 659 and 247 days, respectively, in sunflower and rice brain oil. The corresponding values at 20 °C were 155 and 85 days, respectively. 4. Conclusions Estimation of kinetic parameters of degradation and evaluation of shelf-life is essential for quality assurance of functional foods. The kinetic study outlined in this paper has shown that the stability of lutein in vegetable oils depends on temperature and oil type. Lutein is more stable in sunflower oil, but the values of half-lives are sufficiently high even in rice bran oil. This clearly supports the possibility of utilizing the commercially available lutein preparations to fortify vegetable oils or oily foods. 5. References Bone R.A., J.T. Landrum and L.M. Friedes, 1997, Exp. Eye Res. 64, 211. Chan H.W.S., 1987, Autoxidation of Unsaturated Lipids. Academic Press, London. Codex Alimentarius Commission, 2001, Report of the 17th Session of the Codex Committee on Fats and Oils, London. Henry L.K., G.L. Catignani and S.J. Schwartz, 1998, J. Am. Oil Chem. Soc. 75, 823. Kirschfeld K., 1982, Proc. R. Soc. Lond. B 216, 71. Koca N., H.S. Burdurlu and F. Karedeniz, 2007, J. Food Eng. 78, 449. Krinsky N.I. and K.J. Yeum, 2003, Biochem. Biophys. Res. Commun. 305, 704. Landrum J.T., R.A. Bone and C. Herrero, 2002, Astaxanthin, β-cryptoxanthin, lutein and zeaxanthin, in: Phytochemicals in Nutrition and Health, CRC Press, Boca Raton. Lavecchia R. and A. Zuorro, 2006, Chem. Technol. AIJ 1, 80. Lee M.T. and B.H. Chen, 2002, Food Chem. 78, 425. Mortensen A. and L.H. Skibsted, 1997a, FEBS Lett. 417, 261. Mortensen A. and L.H. Skibsted, 1997b, Free Radic. Res. 27, 229. Mortensen A., L.H. Skibsted and T.G.Truscott, 2001, Arch. Biochem Biophys. 13, 385. Palozza P. and N.I. Krinsky, 1992, Methods Enzymol. 213, 203. Porter N.A., S.E. Caldwell and K.A. Mill, 1995, Lipids 30, 277. Snodderly D.M., 1995, Am. J. Clin. Nutr. 62, 1448.