Photosynthesis - Schooner Chantal
advertisement

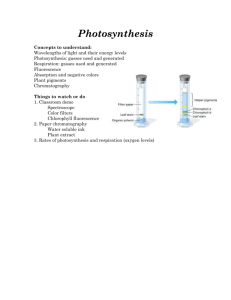
Photosynthesis By Krista Granieri (adapted for COA) Activity 1: The Effect of Light Intensity on the Rate of Photosynthesis Plants and other photosynthetic organisms produce a 6-carbon sugar called glucose (C6H12O6). They use glucose to make structural components, like cell walls, as well as for energy storage. In order to produce glucose, they need a lot of the starting material, carbon dioxide (which has one carbon per molecule). As with any anabolic reaction, the building of glucose molecules from carbon dioxide requires energy. The energy required to put molecules of CO2 together and build them into glucose molecules is obtained from sunlight. Water is another component that is required for this energy transformation to take place. Oxygen is a by-product of the reactions. The overall equation for photosynthesis is: CO2 + H2O C6H12O6 (Glucose) + O2 The process of photosynthesis occurs in two separate sets of reactions. The first set are called the light reactions. This is when the organism uses sunlight and water to obtain and store the energy needed to complete photosynthesis. The sunlight energy is transformed and stored as chemical energy in the form of ATP and NADPH. This process is sometimes referred to as the “energy fixing” reactions. The second part of photosynthesis is called the dark reactions, since light is not required for this part of the process. This is when the organism uses the ATP and NADPH that was generated during the light reactions to construct glucose molecules out of carbon dioxide. This part of the process is sometimes referred to as the “carbon fixing” reactions. Since photosynthesis is a series of chemical reactions occurring inside of cells, it is obviously far too small for us to observe, even with a microscope. If we want to “observe” photosynthesis, we must do so indirectly by observing the usage of the starting materials or the production of the final products. You can do this at home by observing the way your plants use up the water you put into the pots. You have probably also noticed that the plants keep getting bigger. That’s the glucose being produced and used as structural components of the plants like stems, leaves, shoots, etc. In lab, we want to be able to observe photosynthesis more immediately (watching plants use up water can take days). Also, we want to be able to measure this process and see what effect, if any, the intensity of light has on this process. In order to re-create photosynthesis in the lab we must provide all the required components. Materials: Lamp with 200W+ bulb (sunlight) 1.5% sodium bicarbonate solution (carbon dioxide and water) 2- sprigs of Elodea-about 10cm each (plant) You will also need: Meter stick 2- manometer apparati (see diagram) Heat sink (large beaker filled with cold tap water) Razor blade Procedure: 1. Assemble the manometers (see diagram) a. Label the test tubes #1 and #2 b. Cut two very green springs of Elodea about 10cm long and place them both into tube #1- upside down with the cut end up. c. Tube #2 is a control. LEAVE IT EMPTY. #1 #2 Do this part over the sink: d. Completely fill both tubes with the 1.5% sodium bicarbonate solution. e. Cover the tube with your thumb and gently shake to remove bubbles, then refill again to the top with the sodium bicarbonate solution. f. Insert a stopper into each test tube and then place into test tube rack. g. Fill each syringe about half full of water, turn it upside down and push until all the air is removed. Return to your desk: h. Insert the half-filled syringe into the top the needle in the stopper and gently twist to seal tightly. Use caution: the needle protrudes through the stopper. i. Insert pipette into its holder in the rubber stopper. j. Make sure that all the fittings are air tight. 2. By moving the syringe plunger, you can now adjust the water level in the pipettes. Set the level in both pipettes to the 0mm mark on the rulers. Complete the set-up of the photosynthetic system: 3. Place the test tube rack and the heat sink (beaker of water) on the desk next to each other. 4. Use the meter stick to measure and place the light bulb on the desk 45cm away from the manometers. Be sure to put the heat sink in between the bulb and the manometers. 5. Turn on the light and wait 5 minutes to allow the system to stabilize. Begin the experiment: (get your data table ready) 6. After the 5 minute stabilization period, use the syringe plunger to reset the water levels in the pipettes to the 0mm mark. 7. Immediately record the time as the start time in your data table. 8. Allow the experiment to run for 10 minutes and then observe the water levels in the pipettes and record the change in each tube in mm in your data table. 9. Repeat the experiment with the lamp at 30cm and then again at 15cm. Be sure to reset the plunger to the 0mm mark on both tubes before each new run. Analyzing the Results: To determine the amount of oxygen produced as a result of photosynthesis, you must use the data from the control tube (#2). Some amount of oxygen was no doubt produced just as a result of the mixing of the bicarbonate and water. 1. Subtract the mm of oxygen produced in tube #2 from the mm of produced by the Elodea in tube #1. 2. Enter this calculation as the “corrected readings”. 3. Divide the mm of oxygen by the number of minutes. Record this in the last column. Communicating the Results: Show how the intensity of light affects the rate of photosynthesis by creating a graph. 1. The horizontal axis (x-axis) should show the distance from the light in cm. 2. The vertical axis (y-axis) should show the oxygen created in mm/min. Activity 2: Chromatographic Separation of Photosynthetic Pigments The process of photosynthesis is possible because of various pigment molecules present in the thylakoid membranes where the light reactions occur. The pigment molecules are closely associated with the electron transport chain used to produce NADH and ATP. These pigments molecules essentially act as antennae, attracting and trapping photons of light. Each type of pigment absorbs light at a different wavelength. This allows photosynthetic organisms to utilize light efficiently. The primary type of pigments used by plants are called chlorophylls. These absorb light in the blue and red wavelengths and reflect light in the green wavelengths. There are also carotenoids, xanthophyll and phycobiliproteins (phycobiliproteins are found in algae). The color that we experience when we observe an object is created by the wavelengths of light that are not absorbed and thus, reflected by the pigment molecules in the object. The color we see is the wavelengths of light that are being reflected into our eyes. Therefore, when we see a green plant, it is green because photosynthetic pigments tend not to absorb light in the green wavelengths. Because these pigment molecules are different sizes and have different chemical properties, we can separate them using a process called paper chromatography and visualize them individually. Absorption spectra of the various photosynthetic pigments. Chromatography is a process used to separate mixtures of substances into their individual molecular components. All forms of chromatography work on the same principle. They all have a stationary phase (usually paper or gel) and a mobile phase (a solvent). The mobile phase flows through the stationary phase and carries the components of the mixture with it. Different molecules within the mixture will travel at different rates depending on their particular chemical characteristics. In lab we will use paper as the stationary phase and alcohol-acetone as the mobile phase. As the alcohol-acetone solvent is carried up the paper by capillary action (wicking), the molecules in the pigment mixture will travel with it. The various molecules will move at different speeds depending on how strongly they adhere to the paper versus how soluble (attracted) they are in the solvent. Every molecule has it’s own particular adherence/solubility profile. This means that every molecule with travel at a slightly different speed up the strip of paper. The molecules in mixture that have a stronger adherence to the paper will move more slowly than the molecules with high solubility in the solvent. Materials: Paint brush Spinach leaves Mortar & pestle Chromatography apparatus (test tube & cork with hook) Strip of filter paper Test tube rack Procedure: 1. Crush a leaf or two of spinach in the mortar. 2. Set up the chromatography apparatus as in the figure above a. set the test tube upright in the rack- do not put any solvent in it yet b. hook your strip of chromatography paper onto the cork c. put the cork with the paper into the test tube and verify that the length is correct. The paper should be no more than 0.5cm above the bottom of the tube. Cut to the correct length and cut pointed (see diagram). 3. Use paintbrush to paint a thick (~3mm) line of the crushed spinach mixture onto the bottom of the chromatography paper. The spinach mixture should be about 1 cm above the tip of the paper strip. 4. Fill the test tube with alcohol-acetone solvent so that it will just touch the tip of the paper and not touch the line of ground spinach. 5. Place the cork with the chromatography paper into the test tube and let it touch the solvent. Allow it to develop for about 15-20 minutes. 6. Do not allow the solvent to reach the top of the paper strip. Analyzing and Communicating the Results: 1. When the solvent has reached about 1-2cm from the hook, remove the cork and lay the paper out on your lab bench to dry. 2. Dispose of the solvent as directed by your instructor and put away your supplies 3. When the paper chromatograph has dried, you will be able to see several distinct lines of different colors. These are the various pigment molecules found in spinach. Use colored pencils to fill-in the diagram in your lab to look just like your group’s chromatograph. 4. Label the diagram with the names of the pigments you found. Activity 1: The Effect of Light Intensity on the Rate of Photosynthesis DATA TABLE Light Distance cm Time Start Finish Manometer readings Tube #1 Tube #2 Corrected readings Tube #1-tube#2 O2 produced 45 30 15 Light Intensity and the Rate of Photosynthesis mm/min Questions and Discussion: 1. What hypothesis did this experiment attempt to prove? 2. What was the purpose of the heat sink? 3. What was the variable in this experiment? 4. Why did we use sodium bicarbonate in the test tubes? 5. Which tube was the control?___________ 6. Which tube was the experimental tube?_____________ 7. What gas caused the water to move inside the pipette?_____________ 8. Where did this gas come from? 9. Are the results shown in your graph what you expected? Why or Why Not? Explain. 10. As the light intensity increases, the rate of photosynthesis may not always increase along with it. Under what circumstances would the rate not increase as light intensity increases? Activity 2: Chromatographic Separation of Photosynthetic Pigments 1. Draw with colored pencils to show where the various pigments stopped migrating on your chromatograph. Measure the distance traveled for each pigment (from the original line). 2. Then label the pigments on your diagram according to the color chart at the front desk. Distance migrated (cm) Solvent front Pigment mixture Questions and Discussion: 1. Which pigment molecule has the strongest attraction the paper? How far did it travel up the paper strip (in cm)? 2. Which pigment molecule was the most soluble in the solvent? How far did it travel up the paper strip (in cm)? 3. How many different pigment molecules were you able to distinguish? 4. Why do plants have so many different pigment molecules?