Molecular Geometry
Molecular Geometry
Investigating Molecular Shapes with VSEPR
About this Lesson
This activity is intended to give the students opportunities to practice drawing Lewis structures and
then build the corresponding model.
This lesson is included in the LTF Chemistry Module 4.
Objective
Students will:
Draw Lewis structures of selected substances.
Build models using molecular model kits or using toothpicks and gumdrops.
Use the models to visualize the molecular geometry of the molecule and determine the
hybridization.
T E A C H E R
Level
Chemistry
Code
Standard
(LITERACY)
RST.9-10.3
Follow precisely a multistep procedure when
carrying out experiments, taking measurements, or
performing technical tasks, attending to special cases
or exceptions defined in the text.
Translate quantitative or technical information
expressed in words in a text into visual form (e.g., a
table or chart) and translate information expressed
visually or mathematically (e.g., in an equation) into
words.
Write arguments to support claims in an analysis of
substantive topics or texts, using valid reasoning and
relevant and sufficient evidence.
(Literacy)
RST.9-10.7
(LITERACY)
W.1
Level of
Thinking
Apply
Depth of
Knowledge
II
Apply
II
Apply
II
Copyright © 2012 Laying the Foundation®, Inc., Dallas, Texas. All rights reserved. Visit us online at www.ltftraining.org.
P A G E S
Common Core State Standards for Science Content
LTF Science lessons will be aligned with the next generation of multi-state science standards that
are currently in development. These standards are said to be developed around the anchor
document, A Framework for K–12 Science Education, which was produced by the National
Research Council. Where applicable, the LTF Science lessons are also aligned to the Common
Core Standards for Mathematical Content as well as the Common Core Literacy Standards for
Science and Technical Subjects.
Molecular Geometry
Connections to AP*
AP Chemistry:
I. Structure of Matter B. Chemical Bonding 2. Molecular models a. Lewis structures b.
Valence bond; hybridization of orbitals, resonance, sigma and pi bonds c. VSEPR
*Advanced Placement and AP are registered trademarks of the College Entrance Examination Board.
The College Board was not involved in the production of this product.
Materials and Resources
Each lab group will need the following:
gumdrop
paper towels
toothpicks
Additional teacher materials:
Assessments
The following types of formative assessments are embedded in this lesson:
Assessment of prior knowledge.
Visual observations of models built during the laboratory experience.
Guided questions while facilitating.
Copyright © 2012 Laying the Foundation®, Inc., Dallas, Texas. All rights reserved. Visit us online at www.ltftraining.org.
P A G E S
The following additional assessments are located on the LTF website:
Chemistry Assessment: Bonding
2007 Chemistry Posttest, Free Response Question 2; 2008 Chemistry Posttest, Free
Response Question 2; 2010 Chemistry Posttest, Free Response Question 2
AP Style Free Response
Short Lesson Assessment: Molecular Geometry
T E A C H E R
bag, zipper-lock, quart
Molecular Geometry
Teaching Suggestions
Each student pair will need a kit containing 12 gumdrops—two must be the same color and 10 of a
different color. If you have model kits with 4, 5, and 6 holed central atoms, they may also be used.
If you are using model kits it is a good idea to explain the relationship between the number of
holes on the central atom and the sites of electron density in a Lewis structure. Constructing
double bonds should also be discussed.
This lesson is designed to follow an introduction to Lewis structures for covalent compounds.
Students should also have been introduced to the concept of hybridization. During a pre-lab
discussion you should demonstrate the Lewis structures and corresponding geometries for several
of the example compounds in the reference table on the student pages.
Copyright © 2012 Laying the Foundation®, Inc., Dallas, Texas. All rights reserved. Visit us online at www.ltftraining.org.
P A G E S
These regions get increasingly more repulsive moving down the list. You will find a table of basic
VSEPR molecular geometries, along with examples of molecular species that exhibit that
molecular geometry, on the student instruction page. Note that lone pairs are more repulsive than
any of the bonds. This is because they are only influenced by one nucleus rather than two nuclei.
For this reason, lone pairs take up more space and will cause the other bond angles to be smaller.
In general, each lone pair will collapse the bond angle by approximately 2o per lone pair.
T E A C H E R
VSEPR (Valence Shell Electron Pair Repulsion) is a simple model that employs the concept that
electrons, being negatively charged, are repulsive. Therefore, regions of electron densities will
attempt to position themselves as far away from one another as possible. Regions of electron
density are as follows:
Single bond
Double bond
Triple bond
Lone pair
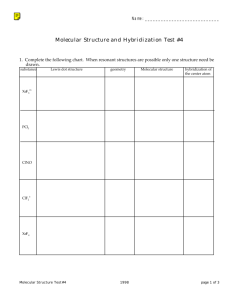
QUESTIONS
2. BF4−
1. CO2
-
F
F
O
C
B
C
F
O
H
F
O
3. H2CO
H
Molecular geometry:
linear
Molecular geometry:
tetrahedral
Molecular geometry:
trigonal planar
Hybridization: sp
Hybridization: sp3
Hybridization: sp2
4. PF5
5. SiH4
F
F
P
6. SeF6
F
H
F
F
F
F
H
Si
H
H
F
Se
F
Molecular geometry:
trigonal
bipyramid
Molecular geometry:
tetrahedral
Molecular geometry:
octahedral
Hybridization: sp3d
Hybridization: sp3
Hybridization: sp3d2
F
F
7. IF4−
8. F2CO
9. XeF4
F
F
F
I
F
F
C
F
O
F
Xe
F
F
F
Molecular geometry:
square planar
Molecular geometry:
trigonal planar
Molecular geometry:
square planar
Hybridization: sp3d2
Hybridization: sp2
Hybridization: sp3d2
10. NO2−
11. O3
-
N
O
O
-
N
O
O
O
O
O
O
Molecular geometry: bent
(2 resonance structures)
Molecular geometry: bent
(2 resonance structures)
Hybridization: sp2
Hybridization: sp2
O
O
12. ClO3−
13. I3−
14. IOF5 (I is the central atom)
F
Cl
O
O
F
I
O
I
I
F
I
F
F
O
Molecular geometry:
trigonal pyramid
Molecular geometry:
linear
Molecular geometry:
octahedral
Hybridization: sp3
Hybridization: sp3d
Hybridization: sp3d2
15. NH2−
-
N
H
H
Molecular geometry: bent
Hybridization: sp3
Molecular Geometry
Molecular Geometry
Investigating Molecular Shapes with VSEPR
The shape of a molecule will dictate many physical and chemical properties of a substance. In
biological systems many reactions are controlled by how substrate and enzyme molecules fit
together. Physical properties of substances, such as solubility and boiling point are also influenced
by molecular geometry.
PURPOSE
In this activity you will draw Lewis structures for a set of molecules and ions. You will then build
the molecules or ions from gumdrops and toothpicks to model the correct VSEPR molecular
geometry and determine the hybridization of the molecules.
MATERIALS
Each lab group will need the following:
gumdrop
paper towels
toothpicks
Safety Alert
Keep the gumdrops on the paper towel at all times. When you are finished, you may
eat the candy if your teacher allows.
PROCEDURE
1. All of the substances on your student answer page are covalent molecules or polyatomic ions.
2. Draw Lewis dot structures in the space provided on your student answer page. Use the VSEPR
theory to predict the molecular geometry of each molecule or ion listed on your student answer
page.
3. Use the gumdrops and toothpicks provided to build each chemical species. Be sure that you
use one toothpick for each pair of electrons on the central atom.
4. Write the hybridization of the orbitals in the space provided for each substance.
Copyright © 2012 Laying the Foundation®, Inc., Dallas, Texas. All rights reserved. Visit us online at www.ltftraining.org.
Molecular Geometry
Molecular Geometry
Investigating Molecular Shapes with VSEPR
VSEPR (Valence Shell Electron Pair Repulsion) is a simple model that employs the concept that
electrons, being negatively charged, are repulsive. Therefore, regions of electron densities will
attempt to position themselves as far away from one another as possible. Regions of electron
density are as follows:
Single bond
Double bond
Triple bond
Lone pair
These regions get increasingly more repulsive moving down the list. The following table is
provided as a reference for basic VSEPR molecular geometries. In the table that follows, M
represents the central atom, X represents the terminal or surrounding atoms and E represents lone
pairs of electrons.
Regions of
Electron
Density
2
3
3
4
4
4
5
5
5
5
6
6
6
Representative
Formula
Example
MX2
MX3
MX2E
MX4
MX3E
MX2E2
MX5
MX4E
MX3E2
MX2E3
MX6
MX5E
MX4E2
CO2
BF3
SO2
CH4
NH3
H2O
PF5
SF4
ICl3
I3 −
SCl6
XeF5+
ICl4−
Molecular Geometry
Hybridization
Linear (180o)
Trigonal planar (120o)
Bent (118o)
Tetrahedral (109.5o)
Trigonal pyramidal (107o)
Bent (105o)
Trigonal bipyramidal
See-saw
T-shaped
Linear
Octahedral
Square pyramidal
Square planar
Copyright © 2012 Laying the Foundation®, Inc., Dallas, Texas. All rights reserved. Visit us online at www.ltftraining.org.
sp
sp2
sp2
sp3
sp3
sp3
sp3d
sp3d
sp3d
sp3d
sp3d2
sp3d2
sp3d2
Molecular Geometry
QUESTIONS
1. CO2
2. BF4−
3. H2CO
Molecular
geometry
Molecular
geometry
Molecular
geometry
Hybridization
Hybridization
Hybridization
4. PF5
5. SiH4
6. SeF6
Molecular
geometry
Molecular
geometry
Molecular
geometry
Hybridization
Hybridization
Hybridization
7. IF4−
8. F2CO
9. XeF4
Molecular
geometry
Molecular
geometry
Molecular
geometry
Hybridization
Hybridization
Hybridization
10. NO2−
11. O3
12. ClO3−
Molecular
geometry
Molecular
geometry
Molecular
geometry
Hybridization
Hybridization
Hybridization
13. I3−
14. IOF5 (I is the central atom) 15. NH2−
Molecular
geometry
Molecular
geometry
Molecular
geometry
Hybridization
Hybridization
Hybridization
Copyright © 2012 Laying the Foundation®, Inc., Dallas, Texas. All rights reserved. Visit us online at www.ltftraining.org.