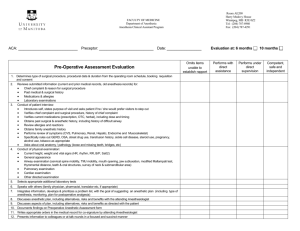
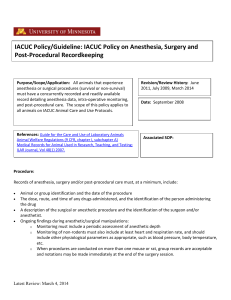
Academy of Surgical Research Certification Study Guide
advertisement

1 Academy of Surgical Research Certification Study Guide 2 Part 1: Introduction The purpose of this study guide is to create an overview of the information in the recommended references for the three certification exams administered by the Academy of Surgical Research. It must be understood that it is these references and not this study guide that are the ultimate source of exam questions and thus topics covered in the references may appear on an exam without being covered in this study guide. However, the majority of the information in the references that might appear on the exams is outlined here. The outline format is designed to allow applicants to use the study guide as a note-taking framework when reading the recommended references. In any work of this size and scope errors have certainly crept into the text. It is hoped that the users of the study guide will make the Academy aware of any mistakes or errors in to allow future corrections. In addition, the purpose of the document is to assist applicants in studying for their exam. Suggestions and requests are welcome in order to make this study guide the most useful reference possible for the applicants. Part 2: Anesthesia Section 2.1: Anesthesia History Prior to 1540: Anesthesia was accomplished with the use of opiates, alcohol, asphyxia, carotid artery compression, physical trauma, and physical restraint when the above didn’t work or weren’t available 1540: Paracelsus makes ether and notes its effects on fowl Overlooked and ignored for the next 300 years 1800: Sir David discovers properties of nitrous oxide 1824: H. H. Hickman demonstrates pain alleviation in dogs using a mixture of nitrous oxide and carbon dioxide 1831: Liebig discovers chloroform 1842: Ether first used for human anesthesia 1844: Dr. Horace Wells discovers the anesthetic properties of nitrous oxide Ignored until 1862 1847: Chloroform used for human anesthesia by Dr. J. Simpson 1847: Chloroform used for animal surgical anesthesia by Flourens 1853: Dr. C. P. Jackson reports extensive use of ether in animal surgery 1854: Dr. G. Dadd advocates humane treatment of animals and application of scientific principles like anesthesia in animals Routinely uses general anesthesia on his surgical patients 1878: Humbert uses IV Chloral Hydrate in the horse 1884: Kohler uses cocaine for local anesthesia in the eye 1898: August Bier produces true spinal anesthesia with cocaine in a dog, himself, and his assistant 3 1900 to late 1920: Ether and chloroform commonly used in small domestic animals Late 1920’s: General anesthesia becomes more accepted as the barbiturates are introduced 1930: Pentobarbital introduced as a general anesthetic 1934: Thiopental introduced 1950: General anesthesia comes into widespread use in large animal surgery due to the introduction of phenothiazine derivatives as preanesthetics This reduces the prolonged and dangerous recovery common without their use 1964: European Association Of Veterinary Anaesthetists formed 1970: American College of Veterinary Anesthesiology organized 1975: American College of Veterinary Anesthesiologists formed 1995: European College of Veterinary Anaesthesia inaugurated Section 2.2: Anesthesia Definitions Anesthesia: Derived from the Greek “anaisthaesia” meaning insensibility, it describes the loss of sensation to the entire or part of the body Local Anesthesia: Loss of sensation in a defined body area Regional Anesthesia: Loss of sensation to a larger though limited body area than described with local anesthesia General Anesthesia: Drug induced unconsciousness that is characterized by controlled reversible depression of the CNS and analgesia Balanced Anesthesia: Induced by a multiple drug approach in which drugs are targeted to specifically attenuate individual components of the anesthetic state (consciousness, analgesia, muscle relaxation, and autonomic reflexes) Dissociative Anesthesia: Induced by drugs that dissociate the thalamocortic and limbic systems and which is characterized by a cataleptoid state in which the eyes remain open and swallowing reflexes remain functional Skeletal muscle hypertonus persists unless a sedative or muscle relaxant has been given Surgical Anesthesia: The stage/plane of general anesthesia that provides unconsciousness, muscular relaxation, and analgesia sufficient for painless surgery The actual depth to achieve these conditions may vary with the invasiveness and painfulness of the procedure Tranquilization: A state of behavioral change, wherein anxiety is relieved and the patient is relaxed, although aware of its surroundings Sedation: State characterized by central depression accompanied by drowsiness where the patient is unaware of its surroundings Narcosis: Druginduced state of deep sleep from which the patient cannot be easily aroused 4 Hypnosis: Condition of artificially induced sleep, or a trance resembling sleep, resulting from moderate depression of the CNS from which the patient is readily aroused Anesthesia Types: Inhalation: Anesthetic gasses or vapors are inhaled in combination with oxygen Injectable: Anesthetic agents are administered IV, IM, SC, IP, and IT Oral and Rectal: Anesthetic agents are administered into the openings of the GI tract Local and Conduction: Anesthetic agents are topically or locally injected into or around a surgical site or a large nerve trunk supplying a specific region Electronarcosis: Passing an electric current through the cerebrum to induce deep narcosis Transcutaneous Electric nerve stimulation: (TENS, TNS, TES) Local analgesia is induced with lowintensity, highfrequency electric stimulation of the skin via surface electrodes Acupuncture: An ancient Chinese system of analgesia using fine needles at predetermined locations Hypothermia: Local or general body temperature is lowered to supplement anesthesia and decrease analgesic drug administration in neonate and cardiovascular procedures Section 2.3: Anesthesia General Issues Anesthesia is a reversible process Anesthetic doses are based on the “average” animal There is no true “average” animal The anesthetist must modify the regimen based on experience and the individual animal’s responses Response to anesthetic agents relies on metabolism, uptake and distribution (pharmacokinetics) of the anesthetic, and preexisting disease or pathology General anesthesia results from interaction of the drug and the CNS General anesthetics may be divided into two categories: Injectable Enters the blood stream for transport to target tissues Requires redistribution Generally are detoxified in the liver and excreted via the kidneys Metabolism based on firstorder kinetics Constant fraction metabolized in a given period Gives less control of the elimination process Inhalation Enters the blood stream from the lungs Primarily eliminated via the lungs 5 Depends on relevant partial pressures and pressure gradients for intake and elimination Gives more control over the anesthetic process due to faster reactions to changes in administration The Perfect Anesthetic Agent: Does not depend on metabolism for its termination of action and elimination Permits rapid induction, quick depth alteration, and rapid recovery Does not depress cardiopulmonary function Is not a tissue irritant Is inexpensive, stable, noninflammable, and nonexplosive Requires no special equipment Unfortunately, no agent fills all of these roles Anesthetists must be familiar with methods of action, dangers, equipment required of the various agents as well as the condition of the animal, desired experimental outcome, and effects of the surgical procedure to determine which agent is the best choice for the procedure Minimum Alveolar Concentration (MAC): MAC is the amount of inhaled anesthetic required to keep 50% of dogs from gross movement in reaction to a painful stimulus at 1 atmosphere Used to test physiological reactions to various stimulus and compounds on susceptibility to anesthetic agents Metabolism: Small animals have a higher basal metabolic rate (BMR) per unit of surface area than larger animals Thus, small animals require larger doses of anesthetic and analgesic agents per kg of bodyweight Basal metabolic rate increases with activity More active animals or during time periods of higher activity require higher levels of the anesthetic agent(s) Disease or pathology may lower metabolic rates Adult and geriatric animals have lower BMRs than adolescent and young adulthood Newborns also have lower BMRs than adolescents and young adults BMR of male is ~7% higher than an equivalent female Fatter animals have slower basal metabolic rates and generally require less anesthetic However, care should be taken as anesthetic agent absorption in adipose tissue may result in less anesthetic agent freely available in the bloodstream May require more anesthetic early on due to absorption into adipose tissue but this may result in longer recovery times due to prolonged discharge of anesthetic agents back into the bloodstream from fat 6 This can occur even after anesthetic agents are no longer being administered as anesthetic agents are still being released into the bloodstream Section 2.4: Stages of General Anesthesia Physiological effects of anesthesia are separated into 4 stages: Stage 1: Stage of voluntary movement Lasts from initial anesthetic administration to the loss of consciousness Tachycardia and hypertension may be present Irregular or increased respirations Patient may hold breath Pupils dilate Struggling may be present as animal becomes ataxic Some analgesic effects may be present at the transition from stage 1 to stage 2 Stage 2: Stage of delirium or involuntary movement CNS becomes depressed Loss of voluntary control Reflexes become more primitive and exaggerated Struggling, breath holding, tachypnea, hyperventilation Cardiac arrythmias may occur Eyelash and palpebral reflexes are present Vocalization Salivation Susceptible to laryngeal spasm Stage 3: Stage of surgical anesthesia Pulse rate returns to normal values Muscles relax Swallowing and vomiting reflexes are lost Divided into 3 or 4 planes depending on the reference Three planes: Light, medium, deep Four planes: I-IV Plane I/Light Eyeball movement ceases Blood pressure returns to normal Strong pulse Begins decrease of respiratory rate and depth Pupils become less dilated Eyeball may rotate Eyelash and palpebral reflex present 7 Slight reaction to surgical manipulation Loses jaw tone Plane II/Medium Surgical anesthesia Bradycardia begins Hypotension increases Capillary refill time begins to slow Palpebral reflex diminishes and disappears Eyeball rotates ventrally Abdominal muscle tone finally lost Jaw tone minimal Pedal reflex absent Dysrhythmia possibility at lowest likelihood Plane III/Medium Deep surgical anesthesia Intercostal and abdominal muscle tone minimal Weak corneal reflexes Diaphragmatic breathing present Profound muscle relaxation present Centered and dilated pupil Bradycardia intensifies Hypotension continues to increase Respiratory rate and depth continue to decrease Plane IV/Deep (Overdose) Dysrhythmia probability begins to increase Respirations slow and irregular Lowered heart rate Cyanosis seen Widely dilated pupil and unresponsive to light Flaccid muscle tone Jaw tone lost Sphincter control lost Stage 4: Beginning to die CNS extremely depressed Respirations slow and cease Heart begins to cease beating Blood pressure at shock level Capillary refill time is greatly increased Pupils relax Dysrhythmia probability at furthest level All reflexes and tone lost Section 2.5: Anesthesia Pharmacokinetics 8 Anesthetic action revolves around plasma concentrations of the anesthetic agent Blood plasma is the carrier of all anesthetic agents to the CNS not administered directly into the CSF Different body parts have different blood supplies and tissue-blood partition coefficients This determines which tissues are easier for the drug to enter and leave Drugs may bind to plasma protein or be distributed in poorly vascularized tissue and reduce the action of the drug After introduction to the blood stream, the drug enters various tissues based on perfusion, capacity for the drug, and the partial pressure gradients Concentration of drug in blood and tissues is generally a factor of partial pressure gradients Partial Pressure Gradients: Molecules are always in motion, moving in random directions This random motion will move molecules from areas of high concentrations into areas of lower concentrations This is not active, simply there are more molecules to randomly move into the lower concentration areas than there are to move back out Eventually, the system reaches equilibrium This is where both sides have an equal number of molecules with both sides having some moving to the other in equal amounts Permeability between the two “sides” will change the number of molecules on either side at equilibrium IV Anesthesia: IV anesthetic plasma concentration falls rapidly Allows quick onset of anesthesia Anesthetic effects are rapid and of short duration because the drug is redistributed into muscle, fat, and vessel poor tissues when drug is rapidly transferred back to the blood from the CNS Duration of action is shorter than with other injectable routes but the drug is present in the body for longer periods than with inhalants Other injectable routes have similar actions once they enter the blood stream 9 The longer time required for IM, SC, IP, etc. administered drugs to enter the bloodstream allows for a more gradual and longer lasting flow of drug into the CNS Allows longer duration of action while taking longer to have effect Doses are typically higher than with IV administration due to the longer time and slower influx of the drug into the blood stream Some anesthetic is already redistributed/metabolized while the drug is still penetrating into the blood stream from the injection site Inhalants are volatile chemicals Molecules are smaller in size than injectable agents Permeate blood stream, tissues, and blood-brain barrier more quickly Primarily exhaled rather than biotransformed Blood-brain barrier: Permeability characteristics similar to cellular membranes Limits penetration of nonlipophillic, ionized, or proteinbound drugs Dependant on partition coefficients, ionization, and protein binding Affected by hypocarbia and hypercarbia Section 2.6: Miscellaneous Anesthesia Issues Patient Examination and Evaluation: Physical Status: Patient’s medical condition (presence or absence of disease) and the overall efficiency and function of organ systems Used to determine what drugs should be administered and approximate dosages Effects on drug uptake, action, elimination, and safety Nervous, cardiopulmonary, hepatic, and renal are the most important Examine patient history Physical exam Attitude, physical condition, conformation, and temperament Palpation, percussion, and auscultation Laboratory exams Blood panels (BUN, Coagulation, etc.) Urine Reference ranges are approximate values With 10 parameter test there is a 40% chance that 1 value will be out of range on a normal animal Use experience for determination Fasting Generally for 12 hours Not advised in small mammals, birds, and neonates which may become hypoglycemic Not recommended in ruminants and horses due to possible rumen shutdown However, distension impairs ventilation and may lead to rupture in the horse 12-24 hours of water withholding may reduce this chance 10 Feeding may lead to increased metabolism as well as possibility of regurgitation Fluids Surgical anesthesia and procedures may lead to dehydration Fluid in body necessary for cell metabolism, intra and extracellular transport, and life itself May be administered IV, SC, orally, or IO (intraosseous) IV fluids should be warmed (~37 degrees C) to prevent hypothermia IO used for severely dehydrated or traumatized patients with poor or inaccessible veins/arteries that need rapid fluid absorption into the blood Common maintenance rate for LRS/Isotonic saline are 10-20 mL/kg/hr 20-30 mL/kg/hr should be used for procedures that involve opening a major body cavity due to dehydration while the organs are exposed Lactated Ringers Solution Closely approximates electrolyte concentrations of extracellular fluid Is isotonic so that it does not induce fluid shifts Since it is isotonic it will not replace lost fluid on a 1:1 basis, will “replace” blood on a 3:1 basis Other solutions such as TisUSol are closer to elctrolytic normals Isotonic (0.9%) and hypotonic (0.45%) saline Do not meet free water and electrolyte needs May lead to dilution of extracellular electrolytes and buffers with excessive use Hypotonic saline with dextrose (2.5%) will act as an isotonic solution Hypertonic (35%) saline Promotes rapid sodium replenishment (hyponatremia) Useful in management of shock (esp. hemorrhagic) 46 mL/kg 7.5% Should be given IV Dextrose (2.550%) solutions Provide source of free water for dehydration treatment Not effective as plasma expanders May be used as a caloric supplement Sodium bicarbonate Treats metabolic acidosis Produces sodium retention Airway Maintenance: For successful anesthesia, an open airway is essential If the airway is blocked, the animal dies If the airway is partially occluded this may cause a decrease in available oxygen to the lungs This may lead to stress and strain to the animal as the body attempts compensation for the lack of enough oxygen Animal positioning is the easiest method to maintain a patent airway Place the animal’s head and torso in a normal anatomical position Positioning alone may not be adequate if: 11 The head must be placed in a poor anatomical position to provide surgical access Procedure’s positioning/restraint does not allow easy access or repositioning (i.e., stereotaxic apparatus) Animal has significant oral/respiratory secretions or potential for stomach/rumen regurgitation In addition, delivery of oxygen or inhalant agents may require use of a face mask or endotracheal tube Face mask Good low tech method to deliver gas to the animal’s respiratory system and not to the general room –Not perfect as the seal is not gastight and there will be some leakage into the room Leakage of inhalant anesthetic agents may be dangerous Endotracheal Tubes Maintain a patent airway Protect airway from foreign material Allow use of positive pressure ventilation Effective delivery of oxygen, inhalant anesthetic agents, and directed test compounds Suction of trachea or bronchi May be made of polyvinyl chloride, silicone, plastic, or rubber Red rubber not advised for use due to a propensity for cracking, difficulty in cleaning, and opacity Cole Endotracheal tubes Uncuffed Have a “shoulder” that leads to a thinner tip Shoulder “seals” against the arytenoid cartilages Should not contact the larynx to avoid pressure on laryngeal cartilages which can cause laryngeal dilation Choice of a proper end diameter (laryngotracheal portion of tube) should create a seal Murphy Endotracheal tubes Cuffed Allows a better seal and does not require precise tip/shoulder placement Overinflation can rupture the cuff or cause pressure trauma to the airway (ischemic injury, mucosal damage, and ultimately tracheal strictures) Recommended pressure of air in cuff 1025 mm Hg May be reinforced or armored with wire/plastic to prevent kinking 12 Will have smaller interior lumens, which can increase resistance to airflow May be reinforced/colored to prevent breaching by laser light during oral procedures Intubation: Basic technique: Induce anesthesia Place animal in an appropriate position, normally sternal recumbency Dorsal recumbency may be useful for swine and primates Open the animal’s mouth Pull the tongue forward and out Apply topical lidocaine spray or liquid to the larynx to prevent laryngospasm (Optional) Alternatively, apply lidocaine jelly to the tip of the endotracheal tube Visualize the epiglottis and glottis A laryngoscope may be helpful Introduce the endotracheal tube into the trachea and advance until the tip is at the approximate level of the thoracic inlet An internal stylet to stiffen the tube may be useful Gauging the appropriate distance to advance the tube in the trachea prior to intubation is quite helpful Secure the tube in place with tape or gauze Gauze may slip in animals with excessive salivation such as ruminants Inflate the cuff if applicable Laryngoscopes Useful for visualizing the glottis and allowing easier intubation It is preferable to use a laryngoscope for better visualization than to show off how practiced one is while injuring the animal because one can’t see Care should be taken not to irritate the epiglottis with the laryngoscope blade Holding the epiglottis with the blade may be traumatic Two main styles of blade Miller: straight Bizarri-Guiffrida: curved 13 Alternate Methods of intubation: Place a thin guide catheter like a urinary catheter into the trachea, thread an endotracheal tube over it into the trachea, and remove the guide catheter After surgically prepping the skin over the trachea, insert a Venicathstyle needle and catheter through the skin and into the trachea, advance the Venicath into the trachea until it is visible in the mouth, thread an endotracheal tube over the Venicath into the trachea, remove the Venicath, and finish placing the endotracheal tube Species specific concerns: Horses, cows, sheep, and goats Usually are eating prior to induction, may require flushing debris from the mouth to allow visualization and to not carry debris into the bronchi Lubrication of the tube with watersoluble lubricants is helpful Visualization may be difficult due to the distance from the mouth to the epiglottis Cats Difficult visualization Pressure under the neck will shift the soft palate and obscure the view Inadequately anesthetized animals may bite down on fingers within the mouth Rabbits Present a very small glottis with poor visualization from the mouth Extending the head back is helpful during blind intubation Carefully observe condensation or listen for breath sounds while slowly advancing tube to allow adequate feedback Following a guide cannula may be useful Rodents Small size makes visualization difficult Using a guide catheter or retrograde guide tube may help Swine Intubation is potentially dangerous due to the false pockets which are highly vascularized pouches outside the trachea If irritated they may bleed profusely Prone to laryngospasm Tube may bind due to sharp angle of route from the mouth into the trachea Gently twisting the tube while advancing is beneficial Lubrication of the endotracheal tube is important As an alternate method for intubation of swine, place pig in dorsal recumbency to which may straighten the pathway for the endotracheal tube Ruminants will regurgitate rumen fluid and saliva Lower head below chest level and/or place a rumen tube and collection bag Rumen tube will also prevent rumen gas distension Thermoregulation: 14 Heating blankets (electric, waterrecirculating, warm air) and/or heated tables should be used to maintain body temperature during surgery Most anesthetic agents depress the thermoregulatory centers and metabolism which leads to accelerated body heat loss Recovery: Small animals Place in lateral recumbency Flip from side to side every 1015 minutes to prevent the lower lung from noninflation, atelectasis, and fluid accumulation Remove endotracheal tube when swallowing reflex returns If animal is not intubated, pull tongue forward to preclude blocking the pharynx Remove water pans until animal is conscious Do not place recovering animals with conscious animals as conscious animals may harm unconscious animals Especially with pigs and rats Large animals (livestock) Remove animal to a padded room/stall Special care should be taken with horses and cows as they can severely harm themselves while struggling during recovery Oxygen and suction should be readily available Ruminants should be maintained in sternal recumbency and have their head held higher than their chest to prevent regurgitation and aspiration of rumen contents Alternatively, with endotracheal tube present and cuff inflated, extend head forward and down Section 2.7: Anesthetic Issues with Disease and Pathology: Cardiovascular dysfunction: Most preanesthetic and anesthetic agents cause CV depression Animals with cardiovascular dysfunction are more prone to fluid overload and arrythmias They may also have a decreased cardiac reserve Pulmonary dysfunction: Most preanesthetic and anesthetic agents cause pulmonary depression Pulmonary dysfunction may be caused by diaphragmatic hernia, pneumothorax, hydrothorax, pneumonia, pulmonary edema, atelectasis, obstruction This condition will require the anesthetist to perform a careful balancing act between lowering doses, primarily preanesthetic drugs, to limit further compromise while also preventing anxiety which will compromise the pulmonary system Intubation and ventilation are key Rapid induction of anesthesia following sedation may be required to permit adequate airflow via intubation 15 Positive pressure ventilation best achieved with inhalation anesthetics Wariness with nitrous oxide is warranted due to its effects on pneumothorax Neurologic disease: Usually refers to spinal cord and to a lesser extent intracranial disorders Intracranial Pressure (ICP) and Cerebral Blood Flow (CBF) are regulated in conscious animals Anesthetized animals may lose this regulation, leading to increases in either of them ICP and CBF increases in head trauma patients may cause further damage Increases may lead to respiratory center depression Most injectable anesthetics lower ICP and CBF Inhalation anesthetics commonly raise ICP and CBF Nitrous oxide causes the greatest increases Renal disease: All anesthetic agents are likely to decrease the rate of filtration Stress also lowers the rate of filtration Effects may terminate at the conclusion of surgery or can linger for days May reduce elimination of anesthetic agents or even increase plasma concentrations due to going acidotic Increases of potassium (K+) which are already present may be elevated in serum to the point of being lifethreatening K+ increases may be seen on the ECG in a peaked T wave, prolonged PR interval, wide QRS complexes, and loss of P wave Chronic renal failure may lead to anemia Rupture of urinary bladder Animal may become hyperkalemic, hyponatremic, hypochloremic, and acidotic An emergency, lifethreatening situation Serum K+ increases Hepatic disease Injectable preanesthetics and anesthetics may cause problems, especially acepromazine, thiobarbituates, and the alpha2adrenergic agents Propofol, ketamine, and inhalation anesthetics are generally the safest The elimination rate of drugs may be lowered due to reduced liver function May result in reduced coagulation abilities Gastrointestinal disease: Damaged GI tract may release toxins into the blood stream May decrease cardiac function and ventilation (distended stomach) Endocrine disorders May be a result of diabetes mellitus or Addison’s disease Anesthetic regimen is not as important as proper treatment of the condition itself Preanesthetic and anesthetic agents should be selected for the shortest recovery time or easiest reversibility 16 Section 2.8: Preanesthetic Agents and Adjuncts Reasons for use: Calm the patient Induce sedation Provide analgesia and muscle relaxation Decrease airway secretion and salivation Obtund autonomic reflex responses Decrease gastric fluid volume and acidity Suppress or prevent vomiting Decrease anesthetic requirements Promote smooth induction and recovery from anesthesia Anticholinergics Tranquilizers Opioids Alpha2adrenergic agonists Alpha2adrenergic antagonists Tranquilizeropioid combinations Paralytic agents Block certain acetylcholine (neurotransmitter) receptors Reduce secretions (saliva) (antisialogogic activity) Prevent vagal inhibition (bradycardia) and GI stimulation (inhibits intestinal peristalsis) Reduce vagus nerve response (vomiting and laryngospasm) Relieve cholinergicmediated bronchoconstriction and promotes bronchodilation Dilate the pupil and reduce tear secretions Diminish the effect of acetylcholine Treatment of choice for opioid, xylazine, and vagal reflex activity induced bradycardia Atropine Sulfate Decrease oral, respiratory, and pharyngeal secretions Increase anatomic and physiologic respiratory dead space Suppresse vagal influence on the heart Decrease lacrimation Increase incidence of cardiac dysrhythmias and sinus tachycardia in dogs Contraindicated with tachycardia, constipation, and obstruction May cause thick mucus secretions in cats Cats, rats, and rabbits (3360%) may have atropine esterase Will destroy large amounts of atropine and limit effectiveness Overdose may cause dry mucous membranes, thirst, dilated pupils, and tachycardia Types of Preanesthetic and Anesthetic Adjuncts: Anticholinergics: 17 Dogs more susceptible Can be treated with repeated doses (if needed) of 0.02 mg/kg physostigmine to a maximum of 0.5 mg/animal administered IV over several minutes Repeated doses should be stopped at a total dose of 2 mg/animal Minimally effective in sheep and goats Will prolong thiopental anesthesia and even cause reanesthetization in dogs Not recommended in rodents due to their rapid HR Not recommended in ruminants due to increased saliva viscosity, short duration, and the increased incidence of bloat Glycopyrrolate Synthetic quaternary ammonium Decrease volume and acidity of gastric secretions, intestinal motility, and tracheal, bronchial, and pharyngeal secretions Reduce diffusion over bloodbrain or placental membranes Block vagal reflexes Lasts longer than atropine sulfate Prevents ketamine/xylazine associated bradycardia in rabbits Lasts longer than atropine but has a longer onset of action in ruminants Tranquilizers: Relieve anxiety Quiet and calm the animal Decrease anesthetic dosages Reduce vomiting Have no analgesic effects Anesthesia recovery is smoother Reduce histamine release (allergy) Promote skeletal muscle relaxation (benzodiazepine agents) May promote vasodilation (phenothiazine agents) Leads to drop in BP (hypotension) and excessive heat loss May lower seizure thresholds (phenothiazine agents) Act as anticonvulsants (benzodiazepine agents) Benzodiazepine agents have minimal side affects Clinical effects Hanging head Drooping ears Glazed eyes Protruding nictitating membranes Acepromazine maleate Phenothiazine derivative Potent neuroleptic agent with low toxicity Dogs; dose should not exceed 3 mg May reduce or prevent malignant hyperthermia in swine Droperidol Butyrophenone tranquilizer 18 Alphaadrenergic antagonist May prevent epinephrineinduced dysrhythmias Decreases barbiturate doses Primarily used as a component of InnovarVet in a mixture with fentanyl Extremely effective combination Diazepam (Valium) Benzodiazepine Prevents seizures Rapidly passes bloodbrain and placental barriers Should be injected slowly to prevent venous thrombosis and must not be injected in an artery Not recommended for intramuscular admin (painful) High doses cause slight decrease in respiration, BP, and CO and increase HR Relatively low toxicity Shouldn’t be mixed with other agents (precipitation) Cats and dogs does not induce sedation alone Midazolam Benzodiazepine Shorter duration of action and clearance than diazepam May cause behavioral changes in dogs and cats Pacing, vocalization Nonirritating and suitable for IM injection Can be mixed with other preanesthetic agents Flumazenil Reverses CNS action of benzodiazepines Rapid action 24 minutes Reversal is not accompanied by anxiety, tachycardia, or hypertension Replaced aminophylline and physostigmine Opioids: Analgesics that will depress the CNS and lower the required amount of anesthetic agents Will not cause unconsciousness at therapeutic doses Addictive Most are controlled substances Require extensive DEA paperwork Relieve continuous dull pain better than sharp and intermittent pain Morphine sulfate Major pharmacologic effect is analgesia Induces a rapid and marked increase in serotonin synthesis Depresses medullary respiratory, cough, and vasomotor centers Does not affect motor function Decreases BMR and body temp (13o F) Stimulates the vomiting center Reflex centers of spinal cord stimulated 19 Use as a preanesthetic agent is confined almost entirely to dogs Causes variable effects on the brain in other species (excitement) Metabolized in the liver and eliminated in urine Has a dose dependant decrease on bile flow Does not appear to affect ruminants Large doses will provoke “rage” in cats Poor effects on neuropathic pain Nerve lesion or disease Meperidine hydrochloride (Demerol, Pethidine) Analgesic effect 1/10th that of morphine Rapidly excreted Reduces salivary and respiratory secretions Does not cause vomiting Rapid IV administration not recommended (may cause hypotension & convulsions) SC administration 30 minutes prior to anesthesia Has “notable” local anesthetic ability Methadone hydrochloride (Methadone, Dolophine) Synthetic opioid unrelated to morphine Reversed with opioid antagonist Stimulates respiration rate Analgesia lasts 2 to 6 hours Decreases barbiturate dose by ~50% Oxymorphone hydrochloride (Numorphan) Semisynthetic opioid 10 times more potent than morphine Little respiratory or cough depression Decreases barbiturate dose ~3366% Can be used to provide effective epidural analgesia Fentanyl Citrate 250 times more potent than morphine Rapid onset of action Short duration with peak at 30 minutes Depresses respiration and may persist for hours Exaggerates response to loud noises Causes vagalmediated bradycardia unless countered (atropine) Little cardiac output or bloode pressure effects If administered with barbiturates may cause bradycardia, hypotension, and respiratory depression Carfentanil citrate 10,000 times more potent than Morphine May be administered by applying to buccal or nasal mucosa Used primarily for capture of wild animals Sufentanil 20 Thienyl analog of fentanyl 5 to 10 times as potent as fentanyl Elimination halflife is 2 to 2.5 hours May induce bradycardia Provides unpredictable anesthesia, bradycardia, hypoventillation, and poor muscle relaxation in dogs when used alone When combined with potent tranquilizers and glycopyrrolate it is an effective neuroleptanesthesic agent Alfentanil 1/5th to 1/10th as potent as fentanyl Has more rapid onset of action than fentanyl or sufentanil Etorphine hydrochloride Oripavine derivative 80 to 1000 times more potent than morphine (SC) Can be antagonized with nalorphine or diprenorphine Used primarily for the capture of wild animals Butorphanol tartrate (Torbugesic) Synthetic opioid 3 to 5 times as potent as morphine Less respiratory depression than morphine Reaches a “ceiling” where additional doses have little effect Excellent analgesic when combined with xylazine or detomidine (cattle and horses) Antagonizes sedative effects or morphine and oxymorphine Buprenorphine (Buprenex) Partial muopioid agonistantagonist 25 to 30 times as potent as morphine (agonist) Maximum analgesic effect less than morphine Onset of action relatively slow (2030 minutes) Causes respiratory depression Reversed by naloxone and naltrexone IM administration lasts 68 hours Epidural administration lasts 1824 hours Mostly excreted unchanged in feces (proteinbound) Pentazocine lactate (Talwin) 1/3rd as effective as morphine (in people) Minimal CV effects Mild respiratory depressant Mediate sedation and analgesia Produce sedation, muscle relaxation, and analgesia Not potent respiratory depressants Not addictive Act as anticonvulsants Alpha2Adrenergic Agonists: 21 Have a widerange of drug interactions Barbiturate, inhalant, and dissociative anesthetic doses should be lowered when used in combination with Alpha2Adrenergic Agonists Xylazine hydrochloride (Rompun) Acts as a sedative and analgesic Most common sedativeanalgesic in horses and cattle Combined with ketamine acts as a shortterm surgical anesthetic agent May be combined with butorphanol to improve analgesia and sedation Major effects develop in 10 to 15 minutes (IM) or 3 to 5 minutes (IV) Duration of analgesia is less than 1 hour IV bolus causes bradycardia and brief hypotension followed by decrease in CO (1/3) and BP (1/4 to 1/3) IM administration has less drastic effects Has poor efficacy in swine Has a wide margin of safety Dose increase prolongs effects but does not generally increase the degree of sedation May cause emesis in cats and dogs Increases urine output in cattle, horses, and cats Causes mydriasis Excited animals (stress, fear, pain) may evince lowered xylazine effectiveness Painful procedures should be limited to 10 to 15 minutes after the onset of sedation IV overdose or arterial administration may cause seizures and collapse Can produce 2nd degree heart block Relatively harmless arrhythmia originating at or below the AV node May show respiratory depression Has a significant local anesthetic effect Epidural administration creates more profound and longer lasting effects than lidocaine Reduces secretion of insulin in the pancreas Not usually harmful except in dehydrated patients where hyperglycemia can lead to transient osmotic diuresis Will affect blood glucose levels Detomidine Developed for horses and cattle Similar cardiovascular effects to xylazine Commonly combined with opioids for enhanced sedation and analgesia May be used as a preanesthetic or combined with ketamine for anesthesia Medetomidine More potent and longer lasting than the other Alpha2Adrenergic Agonists BP and RR are decreased in a dosedependant manner Alpha2Adrenergic Antagonists: Used as reversal agents for injectable anesthetics 22 Yohimbine Reverses xylazine, ketamine, and pentobarbital combinations when combined with 4-aminopyridine Also shown to work well by itself to reverse xylazine Tolazoline Reverses xylazine Partially reverses some anesthetic drug combinations with xylazine Atipamezole Selectivity ratio is 200 to 300 times higher than yohimbine Rapid IV doses may cause death or severe hypotension and tachycardia May be prevented by slower administration Tranquilizer-Opioid Combinations: Used to provide pronounced sedation and analgesia to allow minor surgical procedures Fentanyl citrate Droperidol (Innovarvet) is the primary example Produces neuroleptanalgesia State of sedation with analgesia sufficient for minor surgery Induces intense analgesic actions with relatively short durations Produces sedation, analgesia, immobilization, respiratory depression, decrease in BP, and bradycardia Provides wide margin of safety and easy recovery Partially reversed with opioid antagonists Paralytic (Muscle Relaxant) Agents: Provide superior muscle relaxation as an adjunct to general anesthesia Allow easier intubation and ventilation, improves cardiovascular anesthetic management, and provide less muscle resistance in long-bone orthopedics and abdominal surgery (reduces anesthesia dose required to minimize tension) DO NOT PROVIDE ANALGESIA OR UNCONSCIOUSNESS Are expressly prohibited for sole anesthetic use by the Guide Usually require mechanical ventilation due to paralyzed diaphragm and intercostals muscles Create more difficult anesthesia management Twitching and respiratory signs for maintenance of anesthesia are minimized When paralytics are being used the anesthetist should monitor HR, BP, ECG, and arterial blood gas to gain information on the animal’s physiologic state An inadequately anesthetized animal will show signs of tachycardia, arrhythmias, hypertension,and acidosis Mydriasis and lacrimation may be present Horses will sweat Anesthesiologist may also monitor the response of a peripheral nerve to an electrical stimulus 23 Interact with some antibiotics, lithium compounds, local anesthetics, barbiturates, quinidine, propanolol, calcium antagonists, diuretics, immunosuppressants, and corticosteroids Guaifenesin (glyceryl guaiacolate) Affects tonic (supportive) muscles to the greatest degree Minimally affects respiratory muscles Differentiates it from other paralytic agents Used during induction and short periods of parenteral and inhalation anesthesia May elevate heart rate Metabolized by the liver and excreted by kidneys May be combined with xylazine, ketamine, thiopental, and pentobarbital Neuromuscular (Blocking) Paralytic Agents Interact with receptors at the neuromuscular junction Interfere with transmission of signal from motor neuron to muscle Divided into depolarizing and polarizing agents Depolarizing Neuromuscular Paralytic Agents: o o o o o o o o Depolarizing agents mimic the action of acetylcholine Not antagonized by acetylcholine Increased amounts of agents AT THE NEUROMUSCULAR JUNCTION will not prolong the block Causes initial depolarization of the end plate but do not depart for minutes to hours Keeps the motor neuron from repolarizing and firing again Succinylcholine Must be refrigerated Rapid onset Provides excellent relaxation Will cause marked fasciculation (twitching) for approximately 30 minutes before muscles relax Large doses, repeat doses, or prolonged administration may create a phase II block Characteristic of nondepolarizing blocks May not be reversible Has several undesirable side effects Initial muscle fasciculation and extensor rigidity may cause muscle pain and stiffness and microscopic muscle damage May cause hypertension, tachycardia, sinus bradycardia, cardiac arrhythmias, and cardiac arrest Rise in intraocular pressure Increased blood serum potassium Action prolonged by pregnancy and hepatic disease Can be potentiated by inhalation and local anesthetics Cats, swine, and ponies are resistant Increases intracranial pressure (cats and dogs) despite thiopental or pentobarbital anesthesia 24 Nondepolarizing Neuromuscular Paralytic Agents: Compete with acetylcholine for receptors Do not cause muscle repolarization Actually prevent it Have a slow onset of action Will have prolonged action if hepatic or renal function is impaired Large doses or prolonged administration time can result in prolonged effects Two divisions: Steroid analogs: pancuronium, vecuronium, pipecuronium, and rocuronium Benzylisoquinoliniums: curare, tubocurarine, metocurine, gallamine, atracurium, doxacurium, and mivacurium Pancuronium Lasts 20 to 30 minutes Acts as a comparatively longacting relaxant Causes increased HR (vagal blockade) Prolonged administration will result in a difficult reversal Metabolized in liver Metabolites have weaker relaxant properties Relies on kidney for elimination Vecuronium More potent and shorter acting than pancuronium Prolonged with renal or hepatic failure Does not affect heart rate unless extremely large doses are given Relatively fast recovery Widely used Pipecuronium Long acting Twice the duration of pancuronium o 2 to 4 times as potent Has a rapid onset of action May be retained in the kidneys for days Does not affect heart rate at up to 100 times the therapeutic dose Rocuronium 20% as potent as vecuronium Less dependant on renal clearance Has rapid onset Increased doses hasten onset but will also prolong the duration of activity Rapid recovery Cumulative doses not noted (cats) Curare (dTubocurarine) Long acting Increases heart rate Metabolized in the liver and excreted by the kidneys Small proportion is excreted in bile 25 Causes vasodilation, hypotension, and tachycardia Metocurine Much improved safety margin over curare Relies on renal excretion with 2% excreted in bile Gallamine Relatively impotent Long acting Consistently produces tachycardia Is the only nondepolarizng relaxant that crosses the placenta Relies on renal excretion Atracurium Must be refrigerated Unstable molecule and breaks down by itself at body temperature An intermediate musclerelaxant Not prolonged by renal and hepatic disorders Widely used Doxacurium Long acting Has no autonomic side effects Mivacurium Lasts slightly longer than succinylcholine and 1/2 the duration of vecuronium Does not have autonomic effects Have little effect if a large dose of these nondepolarizing neuromuscular paralytic agents has just been administered Best used when spontaneous recovery of muscle strength has begun Anticholinerases Edrophonium, neostigmine, pyridostigmine May cause bradycardia, bradyarrhythmias, and various secretions 4 Aminopyridine and Guanidine Causes CNS stimulation (restlessness, confusion, and convulsions) Best combined with acetylcholinesterases Calcium Only partially effective Reversal agents: Section 2.9: Injectable Anesthetic Agents and Adjuncts Injectable Anesthesia: Each agent has relatively specific effects Generally requires a combination of drugs to affect all necessary bodily systems adequately Allows specific physiologic targeting by the choice of anesthetic drugs Inhalant anesthetics increase or decrease all components of anesthesia at the same time 26 Includes side effects Anesthetic agents in general are variable to the individual Doses are a guide, not an absolute Remember, opioids ARE NOT anesthetics Barbiturates: Contain a pyrimidine nucleus Divided into 4 groups based on duration of action: Ultrashort: Hexobarbital, Kemithal, Thiamylal, Thiopental Short: Cyclobarbital, Cyclopal, Pentobarbital, Secobarbital Intermediate: Allylbarbituric acid, Amobarbital, Aprobarbital, Butabarbital, Butallylonal, Hexethal, Probarbital, Propallylonal, Vinbarbital Long: Barbital, Diallylbarbituric acid, Mephobarbital, Phenobarbital Short and ultrashort duration barbiturates are the barbiturates used for clinical anesthesia Intermediate and long duration barbiturates are used for sedation and control of seizures Depress the CNS neurons Barbiturate anesthetics can lead to respiratory depression, central and peripheral cardiovascular depression, decreased blood pressure, decreased basal metabolic rate, lowered body temperature, decreased cerebral metabolic rate, reduced cardiac output, reduced stroke volume, and an increase in heart rate Are hypnotic sedatives Cross cell walls and the placenta Cerebrospinal fluid contains less protein for binding than plasma and hence has lower barbiturate concentration at equilibrium Ultrashort acting barbiturates are redistributed faster, NOT metabolized faster, than the short acting barbiturates Glucose effect: During recovery glucose administration will lead to reanesthetization Dogs are less susceptible Rats and mice are not susceptible IV administration is the preferred route IP administration does not allow accurate dosage control to effect Oral administration is variable and usually used for sedation Administered “to effect” First 1/3 of estimated dose is rapidly injected Slow initial injection may cause excitability Remainder given slowly to effect Recommended administration is through a catheter in small animals Perivascular administration will result in “barbiturate slough” Takes 2 to 4 weeks to heal and will scar Lidocaine or 2% procaine (12 mL) may be injected into the area to prevent vasospasm and change the acidity to help minimize damage 27 Saline may also be infused to further dilute the barbiturate Corticosteroids, NSAIDs and/or the use of hot packs may also help Oxybarbiturates: Phenobarbital Sodium Long-acting Effective anticonvulsant Excreted slowly in urine Tends to be cumulative Pentobarbital Sodium After single IV dose arterial BP decreases and HR increases for 10 to 20 minutes Prolonged anesthesia leads to decrease in systolic BP, stroke volume, pulse pressure, central venous pressure, PaO2, pH, CO, and BT HR, PaCO2, and peripheral resistance increases after 1.5 hours Deep anesthesia depresses renal function Effect produced by high doses may closely resemble those of shock Freely crosses the placenta No longer used in North America for cows and horses May show crying, shivering, running motions, and thrashing during recovery Advised to use tranquilizers for the recovery period Methohexital Sodium (Brevital) Ultrashort acting Contains no sulfur atom Duration of action due more to redistribution than metabolism Main danger of overdose is respiratory failure Recovery is difficult even with preanesthetic sedation Best followed by standard inhalant anesthesia Good drug for induction of anesthesia Thiobarbiturates : Thiopental sodium Produces ~12 different metabolic products 86% excreted in urine within 4 days Initially creates a marked respiratory depression Five minutes post-administration: increase in heart rate, aortic pressure, peripheral vascular resistance, and left ventricular pressure Arrhythmias are accentuated by xylazine, halothane, methohexital and epinephrine Cardiovascular effects are reduced if administered along with a lidocaine bolus Has ultrashort action due to rapid redistribution and localization in fat Thiamylal sodium Normal saline suggested as a diluent Solutions stable for up to 14 days Less cumulative than thiopental Tolerance to repeated injections not noted More arrhythmogenic than thiopental 28 Less cardiovascular effects than thiopental Single bolus provides ~15 minutes of surgical anesthesia May be used alone or in combination with guaifenesin in horses and cattle May produce apnea Very safe and nontoxic Nonbarbiturate Anesthetic Drugs: Althesin Combination of the steroids alphaxalone and alphadolone acetate Has little cumulative effect Should not be used along with barbiturates Does not cause damage with perivascular administration Produces good muscle relaxation Additional doses are not cumulative May cause edema of the feet, ears, and muzzle (cats) or allergic reaction consisting of a decrease in blood pressure and wheals at the injection site (dog) Causes violent recovery in horses Chloral Hydrate, U.S.P. May be administered orally or IV/IP if placed in solution Oral admin may cause vomiting Depresses the cerebrum Subanesthetic doses do not affect motor and sensory nerves Is a good hypnotic but poor anesthetic Amount needed for anesthesia is close to the lethal dose Lasts for several hours Has weak analgesic action Fallen out of use for small animals and livestock Perivascular administration is irritating Doses vary extensively Chloralose Produced by heating anhydrous glucose and trichloroacetaldehyde in a water bath to produce alphachloralose Produces minimal CV depression and better maintains active reflexes Provides less depression of neuronal function of the cortex than pentobarbital Valuable for long duration nonsurvival experiments Urethane, N.F. Prepared by heating urea with alcohol under pressure Mutagenic, carcinostatic, and carcinogenic Fallen out of use Magnesium sulfate Globally depresses CNS May be used for euthanasia but only if administered AFTER the animal is rendered unconscious with another agent Metomidate (Hypnodil) A hypnotic with relaxant properties 29 Induces sleep without analgesia Has minimal cumulative effects Etomidate Does not depress CV or respiratory centers or cause histamine release Does not trigger malignant hypothermia in swine Has anticonvulsant properties Venous pain during injection is common (humans) May be a preferred drug for traumatized patients or those with CV or respiratory difficulties Propofol Is not a barbiturate, steroid, or euganol Will support microbial growth and endotoxin production Unused drug should be discarded after 12 hours Rapid uptake into the CNS promotes rapid induction Rapidly redistributed from the brain and metabolized from blood Promotes quick and smooth recovery Has minimal analgesic activity at subanesthetic doses Animal will respond to painful stimuli even under anesthesia Acepromazine “onboard” will decrease the amount needed Decreases intracranial pressure Repeated doses in cats may cause injury to red blood cells Relatively expensive Propanidid Difficult to administer fast enough to cause anesthesia An eugenal Extremely short duration of action Causes severe respiratory depression and hypotension in dogs Tricaine Methanesulfonate (MS222) Used for anesthesia of amphibians and fish by bathing, gill spraying, or injection May be autoclaved Useful for fish, sharks, rays, etc. Dissociative Anesthetics: Used to interrupt ascending transmission from the unconscious to the conscious parts of the brain rather than generalized brain center depression Characterized by a cataleptoid state in which the eyes remain open with a slow nystagmic gaze Provides intense but short duration analgesia Ketamine Most common dissociative injectable Least potent dissociative Clinically available ketamine is actually a racemic mixture (composed of two isomers) Produces doserelated unconsciousness and analgesia Bolus IV injection rapidly crosses the bloodbrain barrier 30 Has a rapid onset of action Termination of effect due to rapid redistribution pH is 3.5, so this may lead to some tissue irritation after IM injection Primary effect at the thalamoneocortical projection system Does not induce seizures and may be an anticonvulsant Analgesic effects greater for somatic pain than visceral pain Increases cerebral blood flow, intracranial pressure, CSF pressure, HR, MAP, and CO Causes transient decrease in respiratory rate Hallucinatory behavior may be evident during recovery Dogs and horses show extensive hepatic metabolism before elimination Cats eliminate most unchanged via the kidney Skeletal muscle tone not affected Often combined with xylazine, guaifenesin, diazepam, medetomidine, or morphine (or derivative) to mitigate excitatory effects Survival rate for “shocky” animals better than with Halothane Telazol Has a wide margin of safety Induction and recovery are rapid and smooth Swallowing, coughing, pedal, corneal, and vomiting reflexes are retained Good muscle relaxant Has lingering analgesic effects May cause increase in HR and respiratory rate MAP may decrease transiently and then decrease May be combined with ketamine and/or xylazine Mixture of tiletamine hydrochloride and zolazepam Tiletamine hydrochloride More effective than ketamine Pharmacodynamics similar to ketamine Causes excitability in rats and mice Large doses produce analgesia and anesthesia May produce transient HR and MAP decreases Overdose will cause hypoventilation and apnea and may increase urine output Zolazepam Does not cause anesthesia or tranquilization in cats Has minimal CNS depressive effects Used to create a mixture that has limited CNS depressant effects Does not cause sufficient muscle relaxation or analgesia in swine when used alone Section 2.10: Physical Methods of Anesthesia Hypothermia 31 Heart, brain, liver, and some other vital organs can survive for longer periods at low temperatures without a portion or all of their blood supply May be general or localized Shivering must be controlled initially by another anesthetic agent Risks profound CNS and vital organ depression May cause severe drops in blood pressure Below 28 degrees Celsius heart muscle may show ventricular fibrillation which will rapidly deplete cardiac muscle stores Heart may be stopped for 30 minutes with cardioplegic solutions Will prolong clotting time Heart-lung bypass may be used to prevent/limit hypoxic brain damage Three methods Surface: Immerse patient in icewater or wrap in an icewater recirculating blanket Body cavity: Pour icedsaline into body cavity (slow) Extracorporeal: Run blood through a heat exchanger Electronarcosis Delivered via electrodes applied to the head Activates opioid or nonopioid control centers in the brain Induction characterized by convulsions unless muscle relaxants are administered Difficult to monitor and of questionable humaneness both for restraint or anesthesia Acupuncture Points may be stimulated with needles, saline injection, electric stimulation, or metal implantation Useful for treatment of chronic pain but not recommended for anesthesia (due to inadequate analgesia and lack of known needle placement sites for specific procedures) Section 2.11: Local and Regional Anesthesia May be used on topical mucous membranes Lidocaine, proparacaine, benzocaine, tetracaine, and butacaine are examples Useful for intubation and eye examination/procedures 20% Benzocaine not recommended for intubation gel Cetacaine is 14% Recent literature suggests that it may cause methemoglobinemia (hemoglobin is oxidized and unable to carry oxygen) May be administered as a solution, in a gel, or as an aerosol May be used as a topical anesthetic Ethylchloride will freeze small areas and cause loss of sensation Potocaine cream penetrate skin EMLA cream (lidocaine and prilocaine) requires 45-60 minutes for penetration (in humans) 32 May be used on muscles exposed at the surgical site May be used as an injectable local anesthetic Most reliable and safest method Lidocaine is the most common Injected SC or ID Mepivicaine or procaine (w/o epinephrine) may be used Epinephrine may cause local ischemia and necrosis May be used as a field block Skin is blocked first and then anesthetic infiltrated into deeper areas May be used as a local nerve block Lidocaine is commonly used Common techniques: Ring block: Inject around the circumference of a limb to provide analgesia for the distal portion Brachial plexus block: Injected medially to the shoulder joint IV Regional: A tourniquet is placed proximally from the surgical site on a limb and lidocaine is administered at the site <90 minutes duration appears safe May be used as an epidural anesthetic Lumbosacral (L7S1) is the common site of epidural administration Excellent for procedures caudal to the umbilicus Useful for cesarean section as it will not depress the infant’s systems Allows conscious animal to care for babies Check for CSF or blood flow into needle prior to injection Blood indicates puncture of the central venous plexus CSF indicates subarachnoid puncture Lidocaine or bupivicaine commonly used May be injected continuously via threaded plastic catheters Opioids injected into epidural space induce profound analgesia Intercostal nerve blocks This affects at least two adjacent intercostal spaces because of nerve supply overlap General muscle blocks Injected into muscles that will be cut, separated, or subject to extensive manipulation during surgery May be used interpleurally Useful for reducing pain after trauma to ribs or a thoracotomy Injected into the pleural space Ineffective if miss-administered into an adjacent space Section 12.12: Inhalation Anesthesia Equipment Anesthetic Delivery Systems: 33 Inhalant anesthetic agents commonly require an anesthesia machine for delivery of the agent Ether, methoxyflurane, and chloroform may be administered via a soaked cotton mask, gauze or placing small animals in a chamber with anesthetic soaked gauze Difficult to control administration and may be dangerous Animals may also be placed in a chamber where anesthetic gas is introduced from an anesthetic machine The anesthesia machine should either be attached to a passive or active scavenging system for collection of waste gasses or the animal should be anesthetized in a ventilated fume hood Two types of anesthesia machines; VOC and VIC systems Vaporizer Out of Circuit (VOC system) The most common style of anesthesia machine Allows more precise control over the concentration of the inhalant gas in the gas mixture administered to the animal Gas Passage Through a VOC System Oxygen is delivered via a tank into the machine through a regulator Using a double circuit ventilator, a Y-piece allows oxygen to pass to the chamber outside the bellows as part of the Driving Gas Circuit Oxygen passes through a flowmeter, allowing adjustment to the flow rate and pressure of oxygen through the system 34 Oxygen then passes through an anesthetic vaporizer where the agent is introduced in known concentrations into the oxygen stream (or other carrier gas) Oxygen/anesthetic mix passes through a unidirectional inhalation valve into an inhalation breathing tube An air intake valve above this valve allows air to enter the system if oxygen flow is disrupted A reservoir bag is attached below to meet peak inspiratory demand and compliance during exhalation Allows assisted or controlled ventilation With a double circuit ventilator the reservoir bag is replaced with a tube leading to the ventilator bellows for collection and pumping of the air through the breathing circuit Oxygen/anesthetic mix is passed into the animal via inhalation or ventilator pressure Animal exhales waste gas Waste gas enters the exhalation breathing tube and overflow gas passes through a popoff valve to an exhaust system Popoff valve allows the system pressure to be adjusted With ventilators, outflow goes to the ventilator for pressure requirements and scavenging system is attached to it The rest of the gas passes through the absorber and CO2 is absorbed before the gas mixture continues the circle Absorber has a manometer above to measure the pressure in the system >20-25 mm Hg is potentially dangerous as it may prevent CO2 absorption Vaporizer In Circuit (VIC system) Vaporizer more passively allows anesthetic to be absorbed into the oxygen stream Does not allow precise control of the agent concentration in the oxygen and thus to the patient Interesting in that the faster the animal breathes (spontaneous ventilation) or with the use of positive pressure ventilation the animal gets an increased amount of anesthetic agent delivered over a given time period Animal begins to recover, breathes faster, gets more anesthesia as the concentration in the gas mixture increases without any operator adjustment No longer produced in the USA 35 Vaporizers: Physics of vaporization Heat energy is required for vaporization Latent heat of vaporization: the number of calories needed to change 1 gram of liquid to vapor This heat requirement causes cooling of the anesthetic during vaporization Vapor pressure of an anesthetic is the partial pressure (PP) of the anesthetic gas above the liquid at equilibrium Vapor pressure varies with temperature Uncontrolled cooling limits the vaporizer’s output Vaporizers are typically made using materials with a high specific heat that supplies heat to the liquid and retards cooling High thermal conductivity also passes heat from the room to the liquid Copper and bronze are most commonly used due to favorable values of heat production and transmission Vaporizers must also compensate for flow, temperature, and pressure Material supplies a “heatsink” Some vaporizers may be heated to provide the higher amounts of heat energy required to vaporize the anesthetic agent; i.e., desflurane Various methods of backpressure compensation are used Mechanical Ventilation: Air enters (A) and compresses bellows and forces air along circuit into patient system (C) Overflow gas from the patient exits via popoff valve (E) to scavenger (B) Adjustment of tidal volume control (F) 36 Bellows may be ascending or descending Ascending is safer as it will collapse if the circuit is opened, descending will continue functioning Operator may not know that there is a problem Collapsed bellows are immediately apparent Intermittent Positive Pressure Ventilation (IPPV): Airway pressure is maintained above ambient pressure during inspiration and falls to ambient pressure to allow expiration Conventional Positive Pressure Ventilation (CPPV): Form of IPPV where a preset tidal volume is delivered at a preset frequency Positive End Expiratory Pressure (PEEP): Endexpiration pressure is maintained higher than the ambient pressure Continuous Positive Airway Pressure (CPAP): Airway pressure is maintained above ambient pressure during SPONTANEOUS respirations Intermittent Mandatory Ventilation (IMV): Used during weaning from a ventilator this allows spontaneous breathing while providing lowered tidal volumes and/or rates The periodic “sigh” that the anesthetist provides is also termed IMV CO2 Absorber Absorbs CO2 before gas passes to the patient side of the circuit Canister should be large enough to contain a gas volume equal to or greater than the patient’s maximum tidal volume around the granules Commonly filled with sodium lime or barium hydroxide lime Lime will expend itself and stop absorbing CO2 May change color or harden Color can change back during storage without allowing further absorption Weight is a better indicator of absorbent saturation than color Lime will also heat up if working properly (heat line) Mapleson Systems Do not use chemical absorbent for CO2 but instead rely on high flow rates Requires more gas and promotes hypothermia and drying of the respiratory system Commonly called nonrebreathing systems Useful for smaller species where the system volume of a standard circuit is too high Two main styles; Magill and Bain Coaxial Systems Magill system Efficient during spontaneous ventilation Fresh gas flow should approximate the patient’s minute volume Volume of the tubing and bag should be equal to or greater than the patient’s tidal volume 37 Bain coaxial system A tube within a tube Popoff valve incorporated in the bag Scavenging Systems: Exposure to waste gasses is a significant concern and health risk Scavenging systems have three parts Gas collecting assembly gathers waste gasses from the system, typically the portion exiting from the popoff valve Interface prevents transfer of pressure changes in the scavenging system to the breathing system Disposal system can be passive or active Both may eliminate waste gasses by venting to the atmosphere or by passing the waste gas through activated charcoal devices Activated charcoal does not absorb N20 Passive gasses rely on the pressure within the breathing circuit from oxygen flow and respiration to push waste gasses through the system Active devices typically have draw fans that provide low negative pressure and actively draw waste gasses through the system 38 Active devices are generally safer and allow less anesthetic gas to be released into the room Section 12.13: Respiration and Ventilation Respiration: the total process whereby oxygen is supplied to and utilized by body cells and CO2 is eliminated by means of concentration gradients Ventilation: movement of gas in and out of the alveoli in the lungs Eupnea: ordinary quiet breathing Dyspnea: labored breathing Tachypnea: increased breathing rate Hyperpnea: fast and/or deep respiration, indicating “overrespiration” Polypnea: rapid and shallow (panting) breathing Hypopnea: slow and/or shallow breathing, indicating “underrespiration” Apnea: transient or longer cessation of breathing Cheyne-Stokes respirations: initial increase in rate and depth, followed by slowing followed by brief periods of apnea Biot’s respirations: sequence of gasping, apnea, and several deep gasps Kussmaul’s respirations: regular deep respirations without pause Apneustic respirations: long, gasping inspirations with several ineffective exhalations Tidal Volume (VT): volume of air inspired or expired in a single breath Inspiratory Reserve Volume (IRV): volume of air that can be inspired over and above the normal tidal volume Expiratory Reserve Volume (ERV): amount of air that can be expired by forceful expiration after a normal expiration Residual Volume (RV): air remaining in the lungs after the most forceful expiration Minute Respiratory Volume or Minute Ventilation (VE): VT times Respiratory Frequency (f) Pulmonary Capacities: Inspiratory Capacity (IC): tidal volume plus the IRV. Amount of air that can be inhaled starting after a normal expiration and distending the lungs to the maximum amount Functional Residual Capacity (FRC): ERV plus RV. Amount of air in the lungs after a normal expiration Vital Capacity (VC): IRV plus VT plus ERV. Maximum amount of air that can be expelled from the lungs after first filling them to maximum capacity Total Lung Capacity (TLC): IRV plus VT plus ERV plus RV. Maximum volume to which the lungs can be expanded with the greatest possible inspiratory effort Ventilation Basics Gas transfer: is the passing of gasses back and forth across a bodily membrane Dependant on a pressure gradient between alveoli and outside atmosphere 39 During inspiration active muscular effort is used to expand the thoracic wall and contract of the diaphragm Expiration is a passive process where the chest wall returns to a normal position Exception is the horse, where abdominal muscle contraction is used (biphasic mode of exhalation) Air flow is dependant on the diameter of the passages Smaller the diameter, the greater the resistance to flow Alveolar air is the critical component. Not equal to minute volume as a large portion fills the respiratory passages This volume is the Dead Space Volume (VD anat) and fills the Anatomic Dead Space Gas is transferred to/from the blood via pressure gradients Under general anesthesia, nasal and pharyngeal musculature relaxes May allow obstruction Section 12.14: Inhalation Anesthesia Differ from all other anesthetic agents because they are administered through and mostly eliminated from the lungs They favor rapid and predictable changes in anesthetic depth Generally require an oxygen source and a patient breathing circuit Latter generally includes an endotracheal tube/face mask, a means of eliminating CO2, and a compliant gas reservoir Historical Inhalant Agents Chloroform May cause liver failure Cyclopropane Explosive Diethyl ether Widely used until ~20 years ago Replaced due to flammability Fluroxene Trichlorethylene All but nitrous oxide are organic compounds Subdivided into: Aliphatic hydrocarbons: Halothane Ethers (2 organic radicals connected by an oxygen atom): Enflurane, Methoxyflurane Found that the lack of an ether group leads to an increased chance of cardiac arrhythmias Reason that all posthalothane agents are ethers Gas and Vapor are the two physical forms of inhaled anesthetics Inhalant Agent Characteristics 40 Gas: an agent that exists in gaseous form at room temperature and sea level atmospheric pressure (nitrous oxide) Vapor: an agent that is gaseous in form while being a liquid at ambient temperature and pressure (all others) Composed of molecules bouncing about at high speed Impact of bouncing molecules on container walls creates a force called pressure Boyle’s law: the smaller the container for a given volume of gas, the more bombardments against the vessel wall in a given area and thus higher pressure Charles’s law: increase in temperature will give more energy to the molecules, so that they move more and thus increase pressure as well Made up of molecules bouncing about at high speed Dalton’s Law of Partial Pressure: Pressures of each gas in a mixture of gasses (called partial pressure) in a mixture added together is the total pressure Quantities of agent can be characterized by pressure, concentration, or mass Commonly use concentration (% of volume) Vapor Pressure All molecules are in motion Some in surface layer will break free and enter the vapor phase (vaporization or evaporation) Will progress to equilibrium These molecules exert force like a gas At any specific temperature there is a maximum amount of vapor that can exist for a given liquid volume (saturated vapor pressure) Primary difference between gas and vapor is that a gas can mix with another gas in concentrations up to 100% Vapor can only exist in concentrations up to the ceiling imposed by the vapor pressure Temperature Heat imparts more energy to liquids, allowing more molecules to escape and thus increasing amount of vaporized liquid Boiling point Temperature where vapor pressure is equal to atmospheric pressure Liquid becomes a gas Solubility Molecules randomly bounce into liquid Continues until equilibrium outside and inside the liquid is present Important because agent solubility in body fluid and tissue determines anesthetic amounts, effectiveness, and pharmacokinetics Partition Coefficients (PC) Difference between the concentration of agent in one liquid/area and another at equilibrium Blood/brain, gas/blood, blood/lipid, etc. Pharmacokinetics 41 Goal is to place an adequate partial pressure of anesthetic in the brain to cause the desired level of CNS depression Agents move down a series of partial pressure gradients (PPG) to the target tissue as well as other tissues Agent is delivered with O2 to the alveoli Must reach appropriate Inspired Concentration IC is a factor of circuit volume Larger the circuit volume, the longer it takes the concentration of gas leaving the vaporizer to be reflected in the animal portion of the circuit Solubility of the agent in the circuit materials will also have an effect Greater the amount of agent delivered to the alveoli (function of respiration rate and pressure) the greater the Alveolar Ventilation Agent moves along PPG into the blood A low blood solubility (PC) requires less agent to reach blood equilibrium and then start passing to other tissues Once in the blood, agent must travel to target tissues Greater vascularity in certain tissue, the greater amount of blood available for agent transfer Function of cardiac output Biotransformation Some agent is metabolized May help in recovery (older agents) May cause acute and chronic toxicities in certain organs Minimum Alveolar Concentration (MAC) is the amount of agent required to produce immobility in 50% of healthy subjects Inversely proportional to potency Refers to % at the alveoli, not at vaporizer or in circuit Gas Cylinders Oxygen Anesthetized animals have higher O2 requirements and have a reduced tidal volume Greater oxygen % in breathed gasses important for adequate supply Can be in supplied in gaseous form or as a highly pressurized liquid CO2 Usually used for rodent immobilization or euthanasia May be mixed with oxygen for less irritation (controversial) Nitrogen Used for powering drills and other equipment Breathing Air Mixture Mix of gasses similar in composition to normal room air Gas cylinder safety O2 and N2O gasses support combustion Sudden release of gas from a broken cylinder may cause injury or propel other pieces of equipment so as to cause injury 42 Broken tank or valve might also create a heavy rocketlike projectile Gas cylinder safety: Tanks are typically unbalanced and possess a small base Tanks should be chained to a wall or cart at all times Regulators should be capped when not attached to gaslines Should be stored away from emergency exits and high traffic areas Note: Tank colors are NOT truly standardized and may vary in color from manufacturer to manufacturer Unlabelled tanks should not be used Inhalant Anesthetics: Nitrous Oxide Tank is filled with liquid N2O under 750770 psi. Some gas is present and is taken off when the tank is opened As gas escapes, more liquid converts to gas until all liquid is removed Thus, pressure of the gas will not change as gas is removed When nothing is left in the tank but gas, the pressure decreases as gas is removed Why weight is better than pressure to gauge amount left in tank MAC is above 100% Cannot deliver sufficient N2O for anesthesia and leave room for oxygen Must be combined with another agent to help lower its required amount Nitrous oxide/oxygen ratio should never exceed 3:1 or insufficient O2 will be delivered Has rapid onset of action due to low blood solubility Has minimal cardiovascular, liver, and kidney effects May transfer to organs or body areas and cause pneumothorax, a blood embolus, a pressure increase in the middle ear, and pneumocephalogram 43 When N2O administration is stopped it floods into the lungs, displacing O2 (diffusion hypoxia) Interferes with CO2 monitoring Halothane Good potency Has high volatility Rapid onset of action Can be delivered as up to 33% of an inspired gas mixture (which can be fatal at the upper level) Causes respiratory depression Depresses myocardial muscle Relaxes vascular smooth muscle Increases cerebral blood flow (vasodilation) Mixed with thymol for stability Thymol is less volatile and will accumulate in devices and causes malfunction unless regularly cleaned Ether The principal inhaled anesthetic agent prior to development of the later nonflammable agents Concentrations used for anesthesia are explosive Patients will release sufficient quantities of ether to be flammable or explosive even after death Highly irritating to the respiratory tract Methoxyflurane Has low volatility and high blood solubility Therefore safe in nonprecision systems Is extensively metabolized Respiratory depressant Has slow onset and recovery Isoflurane Low solubility and high potency Rapid induction and recovery Less cardiovascular effects than halothane Greater safety margin than halothane in rats Potent coronary vasodilator Hyperventilation prior to administration will limit cerebral blood flow increases Minimal chance of renal or kidney injury Desflurane Effects similar to isoflurane More of a respiratory irritant than isoflurane Has lower solubility in blood than isoflurane, halothane, or methoxyflurane Allows rapid equilibrium for quick and precise changes in depth of anesthesia Very rapid induction and recovery Has a low boiling point and thus requires a special heated vaporizer 44 Sevoflurane Effects similar to isoflurane Has lower solubility in blood than isoflurane, halothane, or methoxyflurane Allows rapid equilibrium for quick and precise changes in depth of anesthesia Very rapid induction and recovery Section 2.15: Monitoring Anesthesia Why Monitor? Anesthetic drugs compromise the patient’s physiological homeostasis Monitoring allows the evaluation of physiological feedback from the animal to properly maintain the desired level of anesthesia Allows early notice of trends which may develop into lifethreatening conditions Ultimately gives the anesthetist the information to provides better and more precise anesthesia Proper Monitoring Allows Adequate anesthesia/amnesia Adequate analgesia Adequate immobilization Monitoring Adequate Anesthesia and Amnesia Evaluate muscle tone and reflexes Signs may vary between species and individuals These signs can vary from minute to minute during a procedure Information should be evaluated based on the animal’s reactions and parameters at that instant Previous readings are useful for trends but should not be relied on over “current” readings General anesthetic doses are dependant on the induction drug type, effect, duration of action, and amount administered and the animal’s health condition Adequate Analgesia If the animal is sufficiently anesthetized (unaware/detached from external stimuli) then it may be concluded that sufficient analgesia is present Remember, paralytics may cause an animal to appear anesthetized on cursory exam but still feel pain Light anesthesia may not completely suppress reflexes or spontaneous movement even though analgesia is achieved Adequate Immobilization Based on muscle tone Defined based on needs of the surgical procedure Slight movement that does not interfere with the procedure is acceptable Intraocular procedures, thoracic procedures, longbone fracture repair procedures, and procedures involving extensive laparotomies generally need complete muscle relaxation Neuromuscular blocking agents may be safer than overly deep anesthesia 45 Physiological Effects of Anesthetic Drugs Pharmacodynamic effects may vary but the mechanisms are usually the same Excessive hypotension, bradycardia, arrhythmias, myocardial depression, vasodilation, vasoconstriction, hypoventilation, hypoxemia, etc. Preanesthetic exams (baseline values) should provide clues as to which conditions are already present as well as to alert the anesthetist to potential trouble areas Specific physiologic values should be evaluated by looking at the parameter’s previous trends, combined with other parameters, and with consideration of the patient’s history Pulmonary Monitoring Normal respiratory rates can vary widely Should be evaluated along with tidal volume and respiratory trends May indicate an underlying physiologic change Tachypnea (accelerated breathing) Too lightly anesthetized Too deeply anesthetized Hypoxemia Hypercapnia Hyperthermia Hypotension Atelectasis Drug effects Individual variation Arrhythmic breathing patterns are usually the effect of a medullary respiratory control problem However, some abnormal patterns may be normal in certain species A Cheyne-Stokes (an abnormal breathing pattern that is first shallow and infrequent and then increases gradually to become abnormally deep and rapid, before fading away completely for a brief period. Breathing may be stopped for about 5 to 30 seconds, before the next cycle of shallow breathing begins) pattern is normal in horses but may be a sign of congestive heart failure or other heart or brain disorders Apneustic breathing (inspiratory hold) may be seen in healthy cats, dogs, and most species anesthetized with ketamine Respiratory volume may be estimated visually, by reservoir bag inflation, or by using a ventilator or ventilometer Normal tidal volume is 10-20 mL/kg/respiration Normal total minute ventilation (TMV) is 150-250 mL/kg/min Alveolar minute volume may range from 20-70% of TMV Arterial CO2 and O2 levels may be analyzed by taking arterial blood and measuring with a blood-gas analyzer Venous blood has a tissue bed between it and the lungs where gas exchange takes place and thus is not a good measure of respiratory function 46 Samples should be run immediately or stored in ice water Partial Pressure of CO2 (PaCO2) Measures ventilatory status of the patient Normally ranges between 35 and 45 mm Hg PaCO2 over 60 mm Hg indicates excessive respiratory acidosis and may warrant mechanical ventilation PaCO2 under 20 mm Hg may be a sign of severe respiratory alkalosis and decreased cerebral blood flow Venous CO2 is usually 3 to 6 mm Hg higher than arterial CO2 PaCO2 may be estimated by measuring end tidal CO2 A lowered level is hypocapnia Most often caused by hyperventilation An elevated level is hypercapnia May be caused by: Excessive anesthesia depth Intracranial disease or cervical disease Airway obstruction Thoracic or abdominal restrictive disease Pleural space filling disorder (air or fluid) Terminal pulmonary parenchymal disease Miss-set ventilator settings Hyperthermia Recent bicarbonate therapy Normal level is eucapnia Endtidal CO2 (ETCO2) is the CO2 sample taken from the breathing circuit at the end of an exhalation Accuracy is subject to mechanical factors with the breathing circuit such as volume, dead pockets, tubing diameter, gas flow, etc. Usually somewhat lower than PaCO2 Plateau with a drop to the right may indicate a leak in the circuit as the pressure of inspiration is not held Animals with ETCO2 over 30-40 mm Hg will usually breathe on their own Some hyperventilation is necessary during mechanical ventilation to prevent overbreathing the ventilator Values should be kept between 25 and 30 mm Hg Towards the end of the procedure, the animal should be weaned off the ventilator to restart spontaneous respiration. Gradually slow the rate and/or volume to increase ETCO2 to over 30 to 35 mm Hg During open-skull procedures, values between 18 and 20 mmHg will help prevent brain swelling Cardiopulmonary Monitoring Partial pressure of O2 (PaO2) 47 Measures the oxygenating efficiency of the lungs Is a measure of the oxygen dissolved in the blood and is related to hemoglobin saturation (SaO2) but is not the same thing Usually 80 to 110 mm Hg Pulse Oximetry Measures the percentage of oxygenated hemoglobin and the heart rate Sensor beams infrared light through tissue and records the absorption either of light passing through the tissue to a receiver on the other side (transmission) or reflected back to the sensor (reflectance) Other tissues also absorb light and thus pulse oximeters have a variety of computational ways to interpret the data Is broadly accurate for SaO2 Normally SaO2 is 8090% in spontaneously breathing animals and 95100% in ventilated animals Numbers reflect animal on 100% oxygen SaO2 readings are susceptible to lowering by positional factors (slipping away from tissue, thick tissue, pigment), vasoconstriction, drying of contact surface, and confusion with respiratory artifact HR in anesthetized dogs usually ~60 bpm HR in anesthetized cats usually ~100 bpm Venous Admixture Collective term for all of the ways blood can pass from venous return to arterial supply without being properly oxygenated (hypoxemia) Equipment problem/decreased oxygen inspiratory supply, hypoventilation, bronchoconstriction, atelectasis, inhalation toxicity (diffusion impairment), and anatomic right-to-left shunt Electrocardiogram (ECG) Monitoring heart function Life-threatening situations include Tachycardia: rates higher than 160-180 bpm in cats and dogs Bradycardia: rates less than 60-70 bpm in dogs and 100 bpm in cats Heart Block: loss of or non-P-wave associated QRS complexes Indicate lack of electrical transmission in the heart PVC's: Indicate dangerous arrhythmias Fibrillation: indicates cardiac arrest has begun to occur P Wave is the impulse conduction from SA Node QRS Complex is the ventricular depolarization that precedes contraction T Wave is ventricular repolarization PR Interval lasts from the beginning of atrial excitation to the beginning of ventricular excitation QT Interval is the period of ventricular depolarization and repolarization U Wave Uncertain of origin Relatively uncommon 48 More common in larger species ECG Rates and Arrythmias Bradycardia is slowed heart beat (typically >25% from normal) Tachycardia is accelerated heart beat (typically >25% from normal) Arrythmias are abnormal depolarization, repolarization, and/or contraction of the heart as seen with an ECG Common Arrythmias Sinus Tachycardia P wave seen more closely than normal after the T wave May be described as “T on P” Somewhat common and not typically dangerous except in compromised patients or if it reflects inadequate coronary blood flow Premature Ventricular Contraction (PVC) Commonly caused by hypoxemia and/or hypercarbia and traumatic myocarditis Single PVCs are not usually a problem but a series may result in decreased cardiac output and coronary perfusion which may result in ventricular fibrillation Ventricular Fibrillation A pulseless arrhythmia with irregular and chaotic electrical activity and ventricular contraction in which the heart immediately loses its ability to function as a pump Little or no blood is pumped from the heart Sudden loss of cardiac output, with subsequent tissue hypoperfusion, creates global tissue ischemia; brain and myocardium are most susceptible. Primary cause of sudden cardiac death (SCD) Ventricular Fibrillation (Cont.) Can be counteracted by electrical current (defibrillation) Defibrillation is not always effective and may cause burns and permanent damage to the heart Reflexes Palpebral (blink): begins to be lost at stage 3 of anesthesia Swallowing: many animals will continue to swallow under light anesthesia Endotracheal tubes should not be removed until this reflex returns Pedal Reflex: reaction to pinching a digit or footpad Ear Flick: Particularly useful in cats Corneal: Present until stage 3 plane 4 of anesthesia Laryngeal: May cause laryngospasm in cats Peripheral Perfusion Capillary refill time Measures the time taken for refilling blanched mucus membranes Observe the color of mucus membranes CRT should be 12 seconds and gums (when not pigmented) should be pink Other sites for color are tongue, buccal mucous membrane, conjunctiva of the lower eyelid, and the mucous membranes about the prepuce or vulva Pale membranes indicate poor perfusion, blood loss, or anemia 49 Purple/blue membranes indicate cyanosis Central Venous Pressure Luminal pressure of the intrathoracic vena cava Generally measuring catheters placed via the left jugular vein into the anterior vena cava Don’t touch the endocardium of the right atrium May stimulate ectopic pacemaker activity Normally 0 to 10 cm H2O 15 to 25 in laterally recumbent horses 5 to 10 in dorsally recumbent horses Arterial Blood Pressure Peripheral artery pulse amplitude quality may not closely match central arterial blood pressure Indirect blood pressure Indirect Sphygmometry Monitored with a pressure cuff about an appendage Cuff should be 40% as wide as the circumference of the limb If too tight, the cuff will partially occlude blood flow and artificially lower the value If too loose, the cuff will require increased cuff pressure for contact and the value will be artificially high Cuff should be inflated until it is at a higher pressure than systolic pressure, where it occludes the arteries Doppler piezoelectric crystal Crystal is placed over the artery Transmits energy whose frequency changes based on the movement of the underlying tissue Closely correlates with direct arterial measurements Oscillometric Measures intracuff pressure over several inflations and deflations Provides an averaged reading of systolic pressure, diastolic pressure, mean, and rate The standard style for mechanical blood pressure monitors Direct Blood Pressure Involves a cannula surgically placed into an artery A percutaneous catheter may also be placed into a peripheral artery (pedal, carotid, femoral) Generally attached to a aneroid manometer Fluid filled catheters generally need flushing with heparinized saline or other solution Solid state catheters have no lumen but rather a pressure sensor that transmits the signal electronically No flushing needed Cardiac Output 50 More relevant to systemic perfusion (flow) than a pressure value Reduced by hypovolemia, ventricular restrictive disease, decreased contractility, bradycardia, tachycardia, arrhythmias, retrograde flow (regurgitation), and stenosis Generally obtained via thermodilution Chilled saline of a known temperature and volume is flushed into the artery and the rapidity of the resultant temperature change is measured Temperature Anesthetized animals lose the ability to thermoregulate normally Will lose heat via loss of hair to shaving, the evaporation of prep solutions, evaporation at and chilling of tissues within surgical incisions, and vasodilation caused by anesthetic agents/adjuncts Hypothermia will prolong anesthesia recovery Should be countered with warmed fluids, heating blankets, and towels/wraps Hyperthermia is also possible and dangerous May be due to overheating with heating pads and tables or due to to anesthesia reactions such as malignant hyperthermia in swine Part 3: Analgesia and Pain Management Section 3.1: Definitions Pain: An unpleasant sensory and emotional experience associated with actual or potential tissue damage Suffering: An unpleasant emotional state that is not outwardly represented originating from either a physical or physiological source Distress: The external expression by behavior or emotion of suffering that an observer can see Algology: The science and study of pain Allodynia: Pain caused by a stimulus that does not normally provoke pain Analgesia: Absence of pain in the presence of a stimulus that would normally be painful Analgesic: Drug(s) that induce analgesia Anesthesia: Absence of all sensory modalities, can be local, regional, or total Anesthetics: Drugs that induce regional or general anesthesia Causalgia: Syndrome of prolonged burning pain, allodynia, and hyperpathia after a traumatic nerve lesion, often combined with vasomotor and sudomotor dysfunction and later trophic changes Central Pain: Pain associated with a lesion of the CNS Deafferentation Pain: Pain caused by loss of sensory input into the CNS, as occurs with avulsion of the brachial plexus or other types of peripheral nerve lesions, or caused by pathology of the CNS Dermatome: Sensory segmental supply to skin and subcutaneous tissue Dysethesia: An unpleasant spontaneous or evoked abnormal sensation 51 Hyperesthesia: Increased sensitivity to stimulation, excluding the special senses Hyperalgesia: Increased response to a stimulation that is normally painful Hypoalgesia: Diminished sensitivity to noxious stimulation Hypoesthesia: Diminished sensitivity to stimulation, excluding the special senses Neuralgia: Pain in the distribution of a nerve or nerves Neuritis: Inflammation of nerve cells or nerves Neuropathy: Disturbance of function or a pathologic change in a nerve Nociception: Reception, conduction, and CNS processing of nerve signals generated by the stimulation of nociceptors. It is the physiologic process that when carried to completion results in the conscious perception of pain Nociceptor: Receptor preferentially sensitive to a noxious stimulus or to a stimulus that would become noxious if prolonged Nociceptor Threshold: Minimum strength of a stimulus that will cause a nociceptor to generate an impulse Noxious Stimulus: Stimulus that is actually or potentially damaging to tissue or is of a quality or intensity to trigger nociceptive reactions Pain Threshold: The least experience of pain that a subject can recognize. In most cases is higher than the nociceptor threshold. Relatively constant among species and individuals Pain Tolerance: Greatest level of pain that a subject will tolerate. Varies considerably between species and individuals Pain Tolerance Range: Difference between the pain detection threshold and the pain tolerance threshold Paresthesia: Spontaneous or evoked abnormal sensation. Not painful, as opposed to dysethesia Radiculalgia: Pain along the distribution of one or more sensory nerve roots Radiculopathy: Disturbance of function or pathological change in one or more nerve roots Radiuclitis: Inflamation of one or more nerve roots Reflex: Involuntary, purposeful, and orderly reactions to stimulus. The anatomic basis for the reflex arc consists of a receptor, a primary afferent nerve fiber associated with the receptor, a region of integration in the spinal cord or brain stem and a lower motor neuron leading to an effector organ (skeletal or smooth muscle or glands) Reaction: Combination of reflexes designed to produce a widespread movement in relation to the application of a stimulus. Reactions are mass reflexes not under voluntary control and therefore do not involve the cerebral cortex Response: Willful movement of the body or parts of the body that requires involvement of the somatosensory cerebral cortex Somatic: Describes input for body tissues other than viscera Somatic Pain: General nociceptor based pain Visceral Pain: Differs from somatic pain: Cannot be evoked from all organs (such as the liver and kidneys) Not evoked by burning and cutting Poorly localized 52 May be from nociceptors or other receptors that may have other functions “WindUp” Nociceptors do not demonstrate fatigue with repeated stimulation Rather, they display enhanced sensitivity, lowered threshold of stimulation, and prolonged and enhanced response to stimulation Following a barrage of afferent nociceptor impulses hypersensitization of dorsal horn cells occurs, resulting in an increased rate of discharge (windup) In short, windup allows increased sensitization in nerve tissue to noxious stimulus, a sensitivity that can expand in a receptive field to include distant nerves This creates greater pain from a stimuli than it might otherwise cause Think of it as an avalanche Preventing windup can decrease perceived pain and allow for less analgesic administration Windup can be prevented through the use of local preemptive analgesics and/or the prompt or preemptive use of general analgesics Response to Pain and Injury Stress responses associated with pain are still present unless blocked by epidural or intrathecal anesthetics Peripheral nerve blockage is not as effective Systemic opioids have little effect These responses can also be obtunded or largely prevented by the use of preventative general or local analgesics Nociceptor stimulation of medullary centers results in hyperventilation, increased hypothalamic neural sympathetic tone, and increased secretion of catecholamines and other endocrine hormones Increases cardiac output, peripheral vascular resistance, blood pressure and myocardial oxygen consumption Cortisol and other hormones are released which causes increased blood glucose and ketone levels and increases the rate of metabolism and oxygen consumption Magnitude and duration of these effects parallel the degree of tissue damage These same physiological responses occur in properly anesthetized or unconscious patients but do not result in the sensation of pain due to a nonfunctioning cerebral cortex Pain also causes anxiety and fear These psychological attitudes enhance the stress response Cause cortically mediated increases in blood viscosity, clotting time, fibrinolysis, and platelet aggregation Increase cardiac work, cardiac output, oxygen consumption postsurgery when the cardiac reserve is diminished due to the procedure May lead to intense vasoconstriction which leads to ischemia, tissue hypoxia, and release of substances toxic to the myocardium May result in renal failure Severe posttraumatic or postoperative pain may initiate shock Control of Pain and Injury 53 All of this may lead to retarded postsurgical healing The stress response can be attenuated through adequate pain relief and supportive therapy Initially pain may be controlled with systemic administration of opioids and alpha2adrenergic agents Longterm control may require epidural or spinal administration of these drugs, perhaps combined with local anesthetics In addition, the results of the stress response and tissue reaction to trauma may be treated; Cardiovascular function may be supported by administration of fluids, blood, and blood products Parenteral nutrition may be supplied Splints and bandages may reduce swelling which results in pain as well as immobilizing the damaged area to prevent the animal causing itself pain Corticosteroids and nonsteroidal antiinflamatories may be used Management and Control of Pain Structures involved in the sensation of pain are very similar in humans and animals Reasonable to assume that if something is painful in people, is damaging or potentially damaging to tissues, and/or induces escape or adverse emotional responses in the animals behavior (signs of distress, avoidance behavior, vocalization, changes in body posture) it should be considered painful to the animal If a procedure may result in stimuli likely to cause pain it is appropriate to administer preventative analgesics Accurate selection and dosage will result in the relief of pain without severe respiratory depression Clinical analgesia is not the absence of pain but the clinical reduction of the intensity of pain to a tolerable level Balanced analgesia is the result of administration of analgesic drugs in combination and at multiple sites to induce analgesia by altering more than one part of the nociceptive process Preoperative analgesia is highly effective as it; Prevents spinal neuroplasmic changes (Windup) Suppresses the neuroendocrine response to pain Improves tissue healing and mobility Is the most effective means of controlling postoperative Pain Analgesia may be achieved by obtunding or interrupting the nociceptive process Interrupting the Nociceptive Process Locally The four parts of the nociceptive process: Transduction is the translation of noxious stimuli into electrical nerve impulses at the peripheral nociceptor Reduced with nonsteroidal antiinflamatories 54 Transmission is the propagation of nerve impulses through the nervous system Local anesthetic blockage of peripheral nerves Epidural or subarachnoid analgesics Four parts that may be affected Modulation: Areas where nociceptive transmission is modified by endogenous descending analgesic systems (opioid, serotonergic and nonadrenergic) which inhibit spinal dorsal horn cells Epidural or intrathecal opioids Administration of alpha2agonists Perception is the final conscious subjective and emotional experience of pain Not controllable in animals In humans, meditation and other metal techniques may be used Section 3.2: US Regulations USDA (Animal Welfare Act) Applicable section is from the Regulations and Standards in the Code of Federal Regulations (CFR) in Title 9 C.F.R., Chapter 1, Subchapter A Animal Welfare, Section 2.31 Defines a painful procedure as “any procedure that would reasonably be expected to cause more than slight or momentary pain or distress in a human being to which that procedure was applied, that is, pain in excess of that caused by injections or other minor procedures” Requires that the IACUC ensure that distress and pain will be avoided or minimized to the degree possible USDA (Animal Welfare Act) “Procedures that may cause more than momentary or slight pain or distress to the animals will: (A) Be performed with appropriate sedatives, analgesics or anesthetics, unless withholding such agents is justified for scientific reasons, in writing, by the principal investigator and will continue for only the necessary period of time; (B) Involve, in their planning, consultation with the attending veterinarian or his or her designee; (C) Not include the use of paralytics without anesthesia” Requires veterinary consultation or guidance concerning procedures involving pain and distress In the event that the IACUC committee determines that there are justified scientific reasons for the withholding of analgesic drugs these procedures will be allowed for the minimum necessary period NIH (Guide for the Care and Use of Laboratory Animals) Less specific than the USDA 55 References the book Recognition and Alleviation of Pain and Distress in Laboratory Animals by the Committee on Pain and Distress in Laboratory Animals States that different species express pain and suffering in different ways so personnel must be familiar with species specific and individual expressions of pain Specifies that sedatives, anxiolytics, and neuromuscular blocking agents are not analgesics and must not be used as such PHS (Public Health Service) Implements the Policy on Humane Care and Use of Laboratory Animals (which implements the Health Research Extension Act of 1985 and the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training) Section 3.3: Recognizing and Assessing Pain Difficult to quantitatively measure Requires careful observation and experience in the behavior of the species as well as the individual animal’s behavior Many animals will not exhibit obvious signs of mild to moderate pain Some signs of pain may also be seen in healthy animals Cats hissing and scratching Nonhuman primate aggression Pain may also be seen physiologically Vasoconstriction Increased heart rate, blood pressure, and cardiac output Rapid, shallow breathing Increased plasma concentrations of epinephrine, norepinephrine, cortisol, glucose, glucagon, lipids, ketones, and amino acids Decreased plasma concentrations of phosphorus, magnesium, testosterone, insulin Signs of Mild to Moderate Pain Mouse Partially closed eyelids, rough hair coat, hunched posture, scratching, increased aggression and apprehension, vocalization when handled, selfmutilation Rat Partially closed eyelids, rough hair coat, scratching, increased aggression and apprehension, vocalization when handled, porphyrin staining around eyes and nose Guinea Pig Sunken and dull eyes, respiratory changes, increased timidity and sleepiness, arched back, increased vocalization when handled Rabbit Ocular discharge, constipation or diarrhea, depression, excessive grooming, stretched posture, tooth grinding, dull attitude or aggression Nonhuman Primate Most signs hidden, decreased activity and food and water consumption Dog Decreased alertness, stiff posture, panting, licking, biting, increased aggression and vocalization 56 Cat Increased aggression, decreased food consumption, excessive licking/grooming Pig Changes in gait and posture, increased handling avoidance, increased vocalization Sheep/Goat Lying with extended legs, stamping feet, mild ataxia, depression, restlessness, tooth grinding, increased aggression Sheep are more stoic and less prone to display signs of pain Signs of Severe or Chronic Pain Mouse Weight loss, dehydration, soiled coat, sunken eyes, sunken or distended abdomen, hunched posture, ataxia, hypothermia, decreased vocalization, wasting of back muscles Rat Closed eyes, weight loss, dehydration, soiled coat, sunken or distended abdomen, ataxia, hypothermia, decreased vocalization, wasting of back muscles, incontinence, a recumbent position with tucked head, selfmutilation Guinea Pig Weight loss, scaly skin, dehydration, decreased timidity, unresponsiveness, excessive salivation, increased barbering, loss of righting reflex, decreased vocalization, hypothermia Rabbit Tooth grinding, weight loss, dehydration, wasting of lower back muscles, fecal staining, decreased night feces production, general unresponsiveness Nonhuman Primate Huddled or crouching posture, clenching or grinding teeth, anorexia, weight loss, decreased grooming, decreased socialization, increased aggressive attention from other NHPs Dog Crouched posture, unwillingness to move, depression, increased aggression, vocalization when handled, increased restlessness Cat Hunched, crouching, or stretched posture, increased aggression, anorexia, weight loss, vocalizing, wild escape behavior, unkempt appearance, stiff gait Pig Depression, unwillingness to move, attempts at hiding, anorexia, decreased socialization Sheep/Goat Rolling, frequent looks or kicks to abdomen, falling over, walking backward, rapid and shallow respirations, weight loss, tooth grinding, grunting, vocalization at handling, rigidity, an unwillingness to move Section 3.4: Analgesic Drugs Have as their primary effect the suppression of pain Markedly variable effects among different species for some classes of drugs (i.e., opioids) due to concentrations of receptors that are independent of pharmacokinetics of the drug Six main types of analgesic drugs: Opioids Salicylates Paraaminophenol derivatives Nonopioid, nonsalicylate Local anesthetic agents Alpha2adrenergic agents 57 These are somewhat arbitrary categories and may differ between different references Opioids Morphine, meperidine, methadone oxymorphone, pentazocine, butorphanol, nalbuphine, burprenorphine, fentanyl Induce CNS depression accompanied by miosis, hypothermia, bradycardia, respiratory depression in primates, dogs, rats, and rabbits Induces CNS stimulation, mydriasis, panting, tachycardia, and hyperkinesis in horses, cats, ruminants, and swine Depress respiratory and cough centers May induce nausea and vomiting Mice and rats quickly develop tolerance Well absorbed from GI, IV, SC, IM, IT, and in some cases even transdermally Raise the pain threshold or decrease the perception of pain at the CNS Alter the emotional component of pain to make it more tolerable Drugs of choice for severe, acute pain Most provide analgesia within 30 minutes Have relatively short half-lives Usually on the order of 24 hours Butorphanol: 45 hours Buprenorphine: 812 hours Fentanyl transdermal systems Require application at least 12 hours prior to analgesia need for adequate blood levels Diffusion increased significantly by heat Most are Schedule II Narcotics Salicylates Aspirin is the primary salicylate Initially stimulate the CNS followed by depression Stimulate respiration May induce nausea and vomiting Usually administered orally Half-life dose dependant, larger the dose the longer the half-life due to liver salicyluric acid production limitations Are NSAIDs (Nonsteroidal antiinflammatory drug) Inhibit prostaglandin production at damaged tissue Prostaglandins increases nociceptor sensitivity to stimuli Effective against lowtomoderate intensity pain Not effective against hollow viscera pain Paraaminophenol Derivatives Acetanilid, acetaminophen, phenacetin Similar to aspirin Weak antiinflammatory effects No CV or respiratory effects at therapeutic levels 58 Usually administered orally Are NSAIDs Nonopioid, Nonsalicylate “Everything else” Phenylbutazone, dipyrone, meclofenamic acid, flunixin, carprofen, ketoprofen Better tolerated in humans than aspirin Prolong bleeding times Biotransformed in liver and excreted in urine Local Anesthetic Agents Procaine, lidocaine, mepivicaine, tetracaine, bupivicaine Used as a local anesthetic to prevent nociceptor transmission Bupivicaine has quick onset and 46 hours duration of action Lidocaine has quick onset and short duration of action <2 hours Analgesia is complete at the administration sites Alpha2adrenergic Agents Xylazine, detomidine, medetomidine, and romifidine Sedative hypnotics Are potent analgesics Decrease norepinephrine release Combined with opioids results in enhanced and prolonged analgesia in dogs and cats Part 4: Surgical Regulations and Record Keeping Section 4.1: US Regulations The Animal Welfare Act (AWA) Passed by Congress in 1966 Administered by the United States Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) Regulations administered are called the Animal Welfare Regulations Published in Title 9 of the Code of Federal Regulations The Health Research Extension Act (NIH) (PHS) Taken from policy guidelines established previously by the Public Health Service (PHS) and National Institutes of Health (NIH) in 1985 Contained in the National Regulation Council’s Guide for the Care and Use of Laboratory Animals (commonly referred to as the Guide) and the PHS’ Policy on Humane Care and Use of Laboratory Animals There are minor differences between the two PHS policy specifically implements the “U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training” developed by the Interagency Research Animal Committee Good Laboratory Practices (GLPs) 59 Applies to all research and safety studies funded or reviewed by the FDA EPA has somewhat similar regulations While not directly referencing surgery, these regulations vastly increase the required amount of record keeping Apply to FDA reviewed studies where surgical procedure, device, or compound administration is a study component “If it’s not recorded, it didn’t happen” What Animals are Covered? AWA states that protected species are all mammals (dogs, cats, NHPs, Guinea Pig, hamster, rabbit) used for research, teaching, testing, experimentation, exhibition purposes, or as pets. Exclusions are birds, rats (Rattus) and mice (Mus), and farm animals not used for research purposes However, the Guide covers all species used as laboratory animals Section 4.2: US Accreditations and Guidelines Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC International) Formed in 1965 Offers an accreditation program for qualified institutions AAALAC accreditation is not a requirement for facility operation Standards must be maintained and verified by periodic inspections or accreditation is lost Uses the Guide as part of its standard for program evaluation Guidelines for Animal Surgery in Research and Teaching (GASRT) Required 4 years of effort Originated with the AVMA but stalled at the issue of non-veterinarians performing surgery The AVMA board approved the original report up to the section on personnel and sent the remainder to the American Society of Laboratory Animal Practitioners (ASLAP) Guidelines for Animal Surgery in Research and Teaching (GASRT) ASLAP reviewed and published these guidelines in the September 1993 American Journal of Veterinary Research Emphasized the importance of veterinary participation and oversight in animal research while allowing surgical procedures to be performed by qualified nonveterinary surgeons American College of Veterinary Anesthesiologists (ACVA) Released Suggestions for Monitoring Anesthetized Patients in 1994 Published in the Journal of the American Veterinary Medical Association (JAVMA, Vol. 206, No. 7, 936937.) Covers anesthetic monitoring practices and record keeping 60 Oriented toward clinical veterinarians, however often adopted to cover research animal anesthesia as well Also released the guideline paper Treatment of Pain in Animals Academy of Surgical Research (ASR) Published as the Guidelines for Training in Surgical Research in Animals in the Journal of Investigative Surgery (JIS, Volume 2(3):263268, 1989) Published guidelines for surgery and surgery training on laboratory animals Referenced in the Guide Section 4.3: Surgical Regulations and Guidelines Major and Minor Procedures: Major Procedure AWA and the Guide state that any surgical intervention that penetrates and exposes a body cavity or produces substantial impairment of physical or physiologic functions The Guide gives examples such as laparotomies, thoracotomies, craniotomies, joint replacements, and limb amputations Minor Procedure Guide states that a procedure that does not expose a body cavity and causes little or no physical impairment (such as wound suturing; peripheralvessel cannulation; such routine farmanimal procedures as castration, dehorning, and repair of prolapses; and most procedures routinely done on an "outpatient" basis in veterinary clinical practice). AWA does not define Pain and Distress AWA states that any procedure that could be reasonably expected to cause more than slight or momentary pain or distress in a human is to be considered painful Defined as in excess of that caused by minor procedures such as injections Requires that the IACUC committee ensure that distress and pain will be avoided or minimized Requires veterinary consultation or guidance concerning pain relieving drugs Requires that any procedure that violates the above should be performed with appropriate analgesics, anesthetics, or tranquilizers PHS’s “U.S. Government Principals for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training” echoes the AWA on the use of analgesics Guide is less specific than the AWA References Recognition and Alleviation of Pain and Distress in Laboratory Animals (1992) by the Institute for Laboratory Animal Research 61 States that different species express pain and suffering in different ways so personnel must be familiar with species specific and individual expressions of pain Specifies that sedatives, anxiolytics, and neuromuscular blocking agents are not analgesics and should not be used as such Euthanasia AWA and PHS require that animals that suffer chronic or severe pain or distress should be humanely euthanized at the conclusion of the procedure or, if applicable, during the procedure Anesthetics, Analgesics, Tranquilizers, and Paralytics AWA Same provisions as written for analgesics Prohibits the use of paralytics without anesthesia Guide States that the choice of anesthetic agents should reflect professional judgement Sedatives, anxiolytics, and neuromuscular blocking agents are not sufficient for anesthesia but may be used in combination with anesthetic agents Care must be taken as anesthetic depth may be difficult to assess Recommends monitoring autonomic nervous system changes Recommends that an appropriate level of anesthetic be defined for the procedure without blocking agents Guidelines for Animal Surgery in Research and Teaching (GASRT) Discusses local, regional, and general anesthesia Discusses types of inhalation and injectable anesthetic agents Reaffirms the concern with improper use of muscle relaxants Pre and Postoperative Planning and Care AWA states that pre and postoperative care will be provided in accordance with established veterinary and nursing procedures Guide recommends the “team-concept” as often increasing the likelihood of a successful surgical outcome and that a continuing and thorough assessment of surgical outcomes should be preformed Modification of standard techniques is allowable as long as it does not compromise the well being of the animals Presurgery planning should include input from all members of the surgical team Identify responsible personnel Identify roles and needs Identify required equipment and supplies Identify the facilities involved Discuss preoperative animal healthassessment and postoperative care Investigator and veterinarian share responsibility for postoperative care While recovering from anesthesia, animal should be kept in a clean, dry area and observed often by trained personnel 62 During recovery, attention should be paid to body temperature, cardiovascular and respiratory function, and signs of pain and distress Postoperatively animals should be observed for intake and elimination, signs of postoperative pain, infection, and the appearance of the incision(s) Mandates good care and timely removal of staples, sutures, clips, and bandages Multiple Major Survival Surgery AWA allows only if it is justified for scientific reasons by the principal investigator in writing, is required as a routine veterinary procedure or to protect the health or wellbeing of the animal as determined by an attending veterinarian, or a USDA administrator determines that it fulfills appropriate special circumstances Guide discourages it but permits it when scientifically justified and approved by the IACUC if: Multiple surgeries are related components of a research project or Will conserve animal resources or Needed for clinical reasons Cost savings alone is NOT an adequate reason Requires that the IACUC should pay particular attention to the animal’s wellbeing through continuing evaluation of outcomes PHS policy is similar in tone AAALAC strongly discourages it but permits it if scientifically justified and approved by the IACUC if they are related components of a research project and deemed essential Cost savings alone is NOT an adequate reason Aseptic Technique AWA states that all survival surgery will be performed using aseptic procedures including: Surgical Gloves Masks Sterile Instruments Aseptic techniques Guide states that all major survival surgery will be performed using aseptic procedures including: Clipping of hair at and disinfection of the surgical site Preparation of the surgeon Decontaminated surgical attire Surgical scrub Sterile surgical gloves Sterile Instruments, supplies, and implants All major survival surgery will be performed using good surgical technique including general asepsis, gentle tissue handling, minimal dissection of tissue, appropriate use of instruments, accomplishment os hemostasis, and correct use of suture materials and patterns 63 Minor procedures and those involving rodents may use less stringent measures but still require aseptic procedures and instruments Facilities AWA states that major non-rodent procedures must be conducted only in facilities intended for the purpose and maintained under aseptic conditions and that minor and rodent procedures do not require a dedicated facility but must be performed using aseptic procedures. Guide states that for rodents the facility may be small and simple and should be managed to minimize contamination Guide states that for nonrodents: Common functional components are Surgical support Animal preparation Surgeon’s scrub Operating room Postoperative recovery Areas are commonly separated by physical barriers but may be achieved with distance or timing of appropriate cleaning and disinfection between activities Reduce traffic and personnel Rooms should be designed for ease of cleaning and disinfection and appropriate ventilation Only required equipment and supplies should be kept in the operating room and storage minimized Section 4.4: Surgical Records USDA states that records must be maintained for the annual report and for IACUC functions Guide recommends maintaining the history of surgical procedures and postoperative care, especially for dogs, cats, nonhuman primates, and farm animals “Reading between the lines” good record keeping is useful for the continuing assessment of surgical outcomes ACVA states that all drugs administered must be recorded, noting the dose, time, and route of administration and that monitored variables (minimum of heart rate and respiratory rate) must be recorded every 10 minutes (minimum) during anesthesia GLP regulations require that for surgical protocols performed under GLP regulations, documentation must exist that shows that all parts of the protocol were followed i.e., if the protocol says that Ketamine will be administered IM there should be documentation that this was done Farm animals 64 Guide states that procedures should generally follow the standard guidelines Some minor and emergency procedures may be performed under less stringent conditions Still require the use of appropriate aseptic techniques, sedatives, analgesics, anesthetics, and conditions but may not require the intensive settings, facilities, and procedures detailed in the Guide for other animals AAALAC recommends following the Guide Subject to the same general ethical considerations as any other animal Decisions should be made by the IACUC Agricultural research should follow The Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching Part 5: Surgical Techniques Section 5.1: Tissue Handling Less tissue trauma leads to more rapid healing Handle tissues gently and as little as possible Retractors should be placed to avoid excessive tension May impair blood or lymph flow which changes local physiological state Dry tissue is dead tissue, keep it moist with irrigating saline, LRS, TisUSol, etc. Nonessential material should be removed Wounds with excessive debris should be thoroughly lavaged with an appropriate sterile fluid (isotonic saline, LRS, TisUSol, etc.) to flush them away Hemostasis Bleeding should be stopped whenever possible Excessive bleeding may cause hematomas or increase dead space Hematomas prevent wound apposition and retard healing Blood is a natural food for microorganisms and a large clot will help protect them from the body’s immune system Bacteria inside the clot will be protected Bleeding may be slowed or stopped by applying pressure, clamping, electro/thermocautery, and with various chemicals Excessive pressure may lead to tissue necrosis Dead Space Dead Space is an open area in closed tissue Filled with room air, it prevents tissue apposition, provides a space for blood and other fluid influx, and may harbor microorganisms Section 5.2: Wound Healing Skin and fascia are the strongest tissues but regain tensile strength quite slowly Stomach and small intestine are weak tissues, but heal quickly 65 Wound Healing Phases Phase 1 (day 0 to day 4) Inflammatory response causes an outpouring of tissue fluids, accumulation of cells and fibroblasts, and increased blood supply Leukocytes produce enzymes to dissolve and remove damaged tissue debris Fluids flow into the wound and a scab forms Localized edema, pain, fever, and erythema present Basal cells migrate over the incision from the skin to cover the wound Closure material is the primary source of tensile strength Phase 2 (day 5 to 14) Fibroblasts begin forming collagen fibers in the wound Beginning of the return of tensile strength Fibroblasts migrate toward the wound site Begin forming collagen fibers Tensile strength rapidly increases Lymphatics recanalize Blood vessels bud Granulation tissue forms Capillaries develop Phase 3 (day 14 until done) Sufficient collagen is now laid down to withstand normal stress Tensile strength continues to improve for as long as one year Skin regains 70 to 90% of its original strength Collagen content remains constant but crosslinks with other fibers Scar is formed which grows paler as new vessel construction tapers off Wound contraction occurs over a period of weeks or months Section 5.3: Types of Wound Closure First Intention: Wound edges brought together during closure at the time of surgery Second Intention: Wound is left open and heals from the bottom up Slower than first intention and creates more granulation and scar tissue Third Intention: Wound is initially not closed and remains open until a granulation bed formed, then the granulated tissue is closed using standard techniques Useful in infected wounds where the wound is left open until the infection is controlled Infected tissue should not be closed or it may dehiss Infection is resolved naturally, or with topical and systemic treatments Section 5.4: Classification of Wounds 66 Clean is a standard aseptic surgical wound Clean-contaminated is a clean wound that is contaminated by entry into a viscus resulting in minimal spillage of contents Contaminated is a wound that has become infiltrated with contaminants from lacerations, fractures, gross spillage from the GI tract, or a break in aseptic technique Within 6 hours of initial colonization a wound can be infected Dirty-infected is caused by perforated viscera, abscesses, or a prior clinical infection Ongoing infection at time of surgery may lead to a 400% increase in infection rates Section 5.5: Wound Closure Sutures The ideal suture material: All-purpose, composed of material which could be used in any surgical procedure (the only variables being size and tensile strength) Sterile Nonelectrolytic, noncapillary, nonallergenic, and noncarcinogenic Nonferromagnetic, as is the case with stainless steel sutures Easy to handle Minimally reactive in tissue and not predisposed to bacterial growth Capable of holding securely when knotted without fraying or cutting Resistant to shrinking in tissues Absorbed with minimal tissue reaction after serving its purpose Doesn’t exist! Surgeon should select suture materials for: High uniform tensile strength (quality) Permitting use of finer sizes Suture should be the smallest diameter that will do the job Consistent uniform diameter Sterility Pliable for ease of handling and knot security Freedom from irritating substances or impurities for optimum tissue acceptance Predictable performance Size Generally stated in “oughts”; i.e., 3-0, 5-0, etc. 2-0 is larger than 4-0, 0 is larger than 2-0, etc. Some suture and wire is larger than 0, then numbered 1 and higher 2 is larger than 1, 6 is larger than 1, etc. From smallest to largest: 67 7-0, 3-0, 0, 1, 3, 7, etc. Monofilament suture: Is a single strand Passes through tissue easily, won’t harbor microorganisms Ties easily May be weakened by crushing (clamping in forceps or needle holders) Has more “memory” Continues to hold the shape as it lay in the package Good for percutaneous sutures as the smooth surface is less prone to drawing microorganisms into the tissue Knots may slip over time due to the comparative slipperiness of the suture Multifilament suture: Is a bundle of strands, like rope Affords greater tensile strength, pliability, flexibility, and knot security May harbor microorganisms and “wick” them down the suture Should not be used for percutaneous sutures Absorbable suture maintains strength temporarily but gradually loses tensile strength and is eventually mostly or completely absorbed Nonabsorbable suture will retain tensile strength and not be absorbed Many nonabsorbable sutures (silk) will lose some tensile strength over time Useful for device fixation, areas of extreme tension, slow healing areas, or percutaneous skin sutures Selected for procedures where the suture should be permanent Surgeon’s Knot An initial double throw followed by one or two single throws is generally sufficient Extra throws do not add appreciable strength to the knot and may, in fact, weaken it while adding extra bulk The exception is nylon monofilament sutures, where two successive double throws are useful to prevent slippage Section 5.6: Suture Patterns Simple Interrupted Maintains strength and tissue position if one portion fails Requires more time and suture material Has minimal holding power against stress Horizontal Mattress Tension suture Useful in skin of dog, cow, and horse Rapid and involves less suture material Difficult to apply without excessive eversion Should pass just below the dermis Tightness should be such that the skin edges just meet 68 Vertical Mattress Tension suture Stronger than the horizontal mattress Time consuming and requires more suture material Cross-mattress Tension suture Brings tissue into good apposition Useful in suturing stumps (amputations) Also useful for rib apposition and abdominal muscle closure Gambee or Crushing Useful in intestinal anastamoses Permits minimal leakage May reduce fluid passage through the lumen underneath Crushing is similar to a vertical mattress pattern Simple Continuous Usually used for lines no longer than 5” Involves one diagonal pass and one perpendicular pass Provide minimal tension holding but hold tissue together in good apposition Creates a good seal More prone to failure if any portion is broken Running Both deep and shallow passes advance Regularity more difficult Slightly faster than a simple continuous pattern Weaker than a simple continuous pattern Ford Interlocking More stable in the event of partial failure or breakage Provides greater tissue stability Uses more suture material Lembert Closes hollow viscera Provides inversion and creates a good fluidtight seal Halsted Combination mattress and Lembert pattern Connell Begun with a single inverting vertical mattress suture Continues for the length of the incision Cushing Modified Connell where the needle and suture do not enter the lumen Provides a better fluidtight seal than the Connell pattern Parker-Kerr A single layer of Cushing covered by a single layer of Lembert Used for infected uterine stumps and some bowel closures Provides complete clamping to prevent leakage during suturing 69 Rochester-Carmalt forceps are used to clamp the lumen shut and then slowly withdrawn while placed suture is tightened to prevent spillage of contents Guard Modified Cushing Closes incisions of the rumen, intestine, and uterus Needle does not enter the lumen Starts slightly higher than start of incision Inverting pattern Continuing Everting Mattress Provides increased strength Rapid placement Subcuticular Does not penetrate the surface of the skin Rapid and uses little suture material Used to close the uppermost layer of the skin incision Requires no suture removal Subcutaneous May use simple interrupted, simple continuous, or horizontal mattress Simple continuous is fast and eliminates dead space Quilted Exteriorized skin suture through plastic tubing to resist excessive tension and stress Useful for high-tension closures Far-far, Near-near Tension pattern Overlapping suture pattern provides extra strength but requires extra suture material Near-far, Far-near Tension pattern Overlapping suture pattern provides extra strength but requires extra suture material Mayo Mattress Useful for midline abdominal closures, abdominal hernia repair, and secondary cleft palate repair Bunnell Used for apposing tendons Requires a high degree of closure strength Uses nonabsorbable suture Uses a doublearmed suture Modified Bunnell Used for apposing tendons Requires a high degree of closure strength Uses nonabsorbable suture Uses a singlearmed suture 70 Cerclage Wiring Used for fracture repair Wire/pin placed in the bone center to hold it together Wire winds about the bone under the periosteum Hemicerclage Wire goes through holes drilled in the bone Suture Patterns for Specific Tissues Skin: simple interrupted, horizontal mattress, vertical mattress, continuous apposing or everting Subcutaneous tissue: simple continuous Fascia simple continuous (primary), simple interrupted, vertical mattress, farnear, nearfar, Mayo mattress Peritoneum: simple continuous (two layers), and simple interrupted Very thin and fragile in horse, close muscle instead Vessels: simple interrupted and simple continuous Viscera: direct appositional Cushing suture Muscle: simple continuous, simple interrupted, and horizontal mattress Tendons: Bunnell Bone: hemicerclage and cerclage Part 6: Basic Anatomy and Physiology Functional Organ Systems Skin Muscular Connective Tissue Skeletal Cardiovascular Respiratory Gastrointestinal Nervous Filtration and Excretory Lymphatic Sensory Reproductive Endocrine Covers the body and connects to the mucous membranes of the digestive, respiratory, and urogenital tracts as well as the conjunctivae of the eyelids, the lacrimal duct, and the typanic membrane Acts as a physical barrier between the environment and underlying tissues Assists in regulation of temperature and hydration as well as sensation Consists of the epidermis, dermis, and a subcutaneous layer of adipose tissue Skin 71 Consists of connective tissue bed containing blood vessels, lymphatics, muscles, and nerve endings covered by a stratified squamous epithelium Muscles Three types: Smooth found in the walls of hollow organs and in blood vessels and is under the control of the autonomous nervous system No striations Cannot be voluntarily controlled Spindle shaped and without striations Makes up almost all visceral muscle except cardiac muscle Lines the interior of blood vessels Used for slow, steady contractions Involved in the movement of food and waste products in the GI tract and bladder Involved in vasoconstriction Organized into sheets of fibers surrounded with endomysium and connected by strands of collagen and elastin Cardiac forms the bulk of the heart and is optimized for rhythmic contractions under the control of the autonomic nervous system (ANS) Found only in the heart Striated (but shorter than skeletal) Cannot be voluntarily controlled Works in steady, rhythmic fashion Controlled by the heart’s pacemaker Short and branched Surrounded by endomysium and connected by intercalated discs Skeletal forms bundles surrounded by connective tissue envelopes largely controlled by the somatic nervous system (SNS) Longest muscle cell types Striated Can be voluntarily controlled Made of hundreds to thousands of individual muscle cells called fibers Muscle fibers are enclosed in connective tissue called endomysium Fibers are gathered into bundles called fascicles bound by perimysium Fascicles are bound together by epimysium Origin is the more fixed point of muscle attachment Insertion is the more movable point of muscle attachment In limbs, the insertion is always distal to the origin Movement types Extensor opens a joint or straightens the bone alignment Flexor closes a joint or angulates the bones Adduction moves the extremity toward the median plane (MP) Abduction moves the extremity away from the MP or the axis of the limb Circumduction moves an extremity in a plane represented by a cone Rotation moves a part along its long axis 72 Connective tissue: Provide routes for nerves and blood vessels Envelope, separate, or connect muscles, nerves and blood and lymphatic vessels May blend with the periosteum of bone Periosteum connective tissue covering bone Perichondrium connective tissue covering cartilage Peritoneum a serous membrane largely made of connective tissue Parietal peritoneum covers the abdominal, pelvic, and scrotal cavities Visceral peritoneum covers the abdominal, pelvic, and scrotal organs Connecting peritoneum connects organs to other organs or the parietal peritoneum Common dorsal mesentery peritoneal fold which incorporates most of the abdominal organs Pericardium fibrous sac that envelopes the heart Mediastinum fibrous sheet that separates the two sides of the thoracic cavity and incorporates the heart, thymus, vena cava, and aorta Pleurae serous membranes that line the thoracic cavity and cover the lungs Meninges Protective membranes covering the brain and spinal column Dura mater thick, fibrous, and the most superficial of the meninges Arachnoid membrane delicate and lines the deep surface of the dura mater Pia mater delicate and coats the surface of the brain, spinal cord, nerve roots, and the optic nerve Skeleton: “The framework that the body hangs on” Supports and protects the body Provides attachment points for the muscles and acts as levers for motion Made up of individual bones connected by ligaments and muscles Bones Two types of bone tissue Compact bone: Dense tissue that forms the outer shell of all bones Spongy bone: Filled with pockets and lays in between most compact bone surfaces Types of bones Long bone consists of a shaft (diaphysis) with two extremities (proximal and distal epiphysis) Includes all bones in the limbs except the patella and those of the wrist and ankle Short bone is roughly cubelike, primarily spongy bone surrounded by a thin layer of compact bone Bones of the wrist and ankle Sesamoid bone is a short bone embedded in a tendon or joint capsule i.e., the patella Flat bone is a thin bone with spongy bone called diploe encased in compact bone 73 The sternum, the ribs, and most bones of the skull Irregular bone – bones that don’t fit into the previous categories Vertebrae, pelvis, some skull bones Structure of a flat bone, outside to inside Periosteum, a fibrous tissue connected to the bone by Sharpey’s fibers Compact bone Spongy bone which contains either red bone marrow or a medullary cavity (containing yellow bone marrow) The medullary cavity in spongy bone as well as the internal termination of the compact bone is lined with a fibrous tissue called endosteum General Information Bone acts as a storage site for calcium and phosphorus Bone components produce both red and several types of white blood cells The skull is made of the incisive, nasal, maxilla, zygomatic, lacrimal, frontal, palatine, pterygoid, sphenoid, parietal, occipital and temporal bones Occipital bone has sagittal and nuchal crests Radius and tibia are the main weight supporters of the lower limbs Consists of ~321 bones in the dog 50 separate bones for the skull and hyoid 50 vertebrae 7 sternum and the xiphoid process 26 ribs 90 bones in the forelimbs 96 bones in the hindlimbs 1 Os penis in the male Joints are where bones are joined by fibrous, elastic, and/or cartilaginous tissue Ligament – fibrous tissue connecting bone to bone Tendon – fibrous tissue connecting muscle to bone May be categorized by functionality or structure Function Synarthroses – permits no movement Amphiarthroses – permits slight movement Diarthroses – permits free movement Structure Fibrous bones are joined by fibrous tissue, no joint cavity exists (skull sutures, teeth) and provide little or no movement Cartilaginous bones are united by cartilage, no joint cavity exists (Epiphyseal disc, intervertebral disc), and permits compression or stretching Synovial bones are separated by a joint cavity and permits significant movement Synovial joints may be plane, ball-and-socket, ellipsoidal, hinge, condylar, trochoid, or saddle joints 5 main components of synovial joints: 74 Articular cartilage covering the bone at the joint Has limited regeneration capabilities Articular capsule encloses the joint in an outer fibrous capsule and an inner synovial membrane Hip and knee joints have a fatty pad between the fibrous capsule and the synovial membrane or bone Joint cavity is the potential space within the articular capsule that is filled with a nutrient and waste transporting fluid called synovial fluid Synovial fluid is a lubricant made of hyaluronic acid thinned by interstitial fluid from blood plasma Reinforcing ligaments reinforce and strengthen the joint (intrinsic, extracapsular, intracapsular) Nervous System Brain Composed of cerebrum, cerebellum, and brain stem Cerebrum primary site of cognitive and sensory functions Cerebellum coordinates movement and posture Brain stem source of all cranial nerves except the olfactory nerves and is continuous with the spinal cord Divided into two systems; central nervous system (CNS) and peripheral nervous system (PNS) In reality it is a single integrated system which science has arbitrarily divided CNS consists of the brain and spinal cord Processes sensory information and sends commands PNS is everything else Sensory (afferent) division conveys impulses from sensory organs to the CNS Motor (efferent) division transmits impulses from the CNS to muscles and glands Efferent Division Divided into two systems Somatic Nervous System (SNS) conducts impulses to muscle fibers and allows voluntary control Autonomic Nervous System (ANS) regulates smooth muscles, cardiac muscles, and glands ANS divided into two systems; Sympathetic Division Originates from the spinal cord between T1 and L2 Parasympathetic Division Originates from the brain stem and sacral region of the spinal cord Sympathetic and parasympathetic regulate each other Brain floats in cerebrospinal fluid Contains inorganic ions, protein, sugar, and a few misc.cells Derived from blood and returns to blood stream via arachnoid villi and lymphatics Spinal cord 75 Extends from the brain stem through the vertebral canal Conducts signals to and from the brain Acts as a reflex generator Processes information and discharges commands Has a central canal that carries cerebrospinal fluid Neurons Have a body (soma) that contains the nucleus and cytoplasm Dendrites are small branches extending from the soma that act as receptive sites to conduct electrical signals toward the soma The axon stems from the soma and transmits nerve impulses away from the soma to axonal terminals Axonal terminals conduct signals to muscles, glands, and other nerves Nerves A nerve fiber (neuron) is surrounded by fibrous tissue called endoneurium Nerve fibers are bound into bundles with perineurium to form fascicles Fascicles and blood vessels are bound into nerves by epineurium In the CNS nerves are termed tracts Nerves are highly variable in structure and may differ from these descriptions Cardiovascular System: Heart Made up of 4 chambers Deoxygenated blood enters the right atrium via the vena cava Blood is then pumped through the right atrio-ventricular valve (a tricuspid valve although it consists basically of two cusps) into the right ventricle Blood is then pumped through the pulmonary valve into the pulmonary artery, to the lungs for oxygenation, back through the pulmonary veins, and into the left atrium Blood is pumped through the left atrio-ventricular (bicuspid or mitral) valve into the left ventricle Blood is pumped to the aorta and then throughout the body Heart is kept supplied with blood via the right and left coronary arteries which divide into the various branches (right marginal branch, circumflex branch, left marginal branch, etc.) that supply the heart Acts as the primary pump of the cardiovascular system Contraction involves the Sinoatrial (SA) Node, Atrioventricular (AV) Node, AV Bundle (Bundle of His), right and left branches, and Purkinje fibers SA Node is a pacemaker that depolarizes in a sinus rhythm to begin the contraction of the atria and conducts the signal via the atria to the AV Node AV Node delays the impulse to allow the atria to fully contract and then transmits the signal AV Bundle transmits the signal to the branches which then transmit it to the Bundle of His which is made up of Purkinje fibers Purkinje fibers transmit the signal evenly throughout the ventricles to ensure even contraction in a wringing manner towards the atria 76 Contraction does not require outside stimuli but can be regulated by the ANS Cardiac Cycle Systole is the contraction period of the heart Diastole is the resting period Mid to late diastole involves low blood pressure in the heart and blood passively flowing through the atria into the ventricles AV valves are open Atrial Systole involves the atria contracting Ventricular Systole starts as the atria go into diastole and involves ventricular contraction Momentarily all valves are closed and ventricular pressure rises sharply Early Diastole is where the ventricles begin to relax Cardiac Output Amount of blood pumped out by each ventricle in 1 minute Heart Rate times Stroke Volume Stroke Volume is the volume of blood pumped by the left ventricle for each contraction Heart Sounds “Lupdub” sound results from the valves closing “Lup” is AV valve closure “Dub” is the semilunar valves closing Unusual sounds are termed murmurs Vascular System Distribution of blood The bulk (70%) of the blood is in the venous system with only 10% in the arterial system Blood leaves the heart via the aorta and cardiac arteries Arteries carry blood to smaller arterioles Arterioles carry blood to capillaries Oxygen is supplied to the tissues around the capillaries Capillaries return deoxygenated blood to venuoles Venuoles carry blood to veins Veins carry blood to the vena cava The vena cava returns blood to the heart Arteries and Veins Tunica Intima is the inner surface of the vessel consisting of a single layer of endothelium with a basement membrane Tunica Media is made of smooth muscle and elastic fibers Layer is much thicker and stronger in arteries Tunica Adventitia is made of collagen fibers Capillaries Made of endothelial cells and a sparse basal intima Primary site of oxygen exchange between the blood and body tissue Aorta 77 Carries all oxygenated blood from the heart Extends cranially before making a 180 degree halfcircle to proceed caudally Arbitrarily divided into the ascending aorta, aortic arch, descending aorta (thoracic and abdominal aorta) Arteries Originate from the aorta Main surgical arteries are: Brachiocephalic trunk: Originates at aortic arch and feeds both common carotid arteries and the right subclavian artery Left and right common carotid arteries: Originate at the brachiocephalic trunk and branch to become the internal and external carotid arteries at C1C2 Left subclavian artery: Originates at the aortic arch Right subclavian artery: Originates at the brachiocephalic trunk Subclavian arteries continue as the axial arteries which feed the forelimbs Axial arteries: Continue as the left and right brachial arteries which feed the forelimb Celiac artery: Originates at the abdominal aorta and branches into the hepatic, gastric, and splenic arteries Cranial and caudal mesenteric: Originate at the abdominal aorta and feed the intestines Left and Right renal arteries: Originate at the abdominal aorta and feed the kidneys Left and right external iliac arteries: Branch from the termination of the abdominal aorta and continue as the left and right femoral arteries Veins Return deoxygenated blood from the capillary beds to the heart Main surgical veins are: Left and right femoral veins: Transport blood in the hindlimbs to the external iliac vein Left and right external iliac veins: Continue the femoral veins and join the common iliac when joined by the internal iliac vein Common iliac veins: Connects to the caudal vena cava Portal vein: Collects blood from pancreas, spleen, and all of the GI tract save the anal canal and connects to the liver and thence to the hepatic veins that connect to the caudal vena cava Left and right external jugular: Continues the internal and external maxillary veins and connects to the brachiocephalic veins Cephalic vein: Connect to the brachiocephalic vein and provide flow from the forelimbs and is situated on the lateral aspect of the forelimb Brachial vein: Connecst to the brachiocephalic vein and provide flow from the forelimbs and is situated on medial aspect of the forelimb Left and right subclavian veins: Drain into the cranial vena cava 78 Vena Cava: Collects venous blood and drains into the right atrium and is arbitrarily divided into cranial and caudal vena cava at the diaphragm before it drains into the heart Respiratory System: Transports oxygen from the outside environment to the blood stream Parts of the respiratory system Pharynx: The main passageway from the nose and mouth Larynx: Musculocartilaginous organ that protects the entry to the trachea Parts of the respiratory system Trachea: Flexible, wall of smooth muscle surrounded by Cshaped rings of hyaline cartilage Passageway for air to the left and right principal bronchus Bronchus: Divides into lobar bronchi which then divide into segmental bronchi which further divided into the alveoli Lung: Organ containing the bronchus, bronchi, and alveoli Are passive organs, inflation being due to the diaphragm Each lung lies in an essentially potential cavity called the pleural cavity Is a thin film of moistening fluid Becomes a true cavity in a pneumothorax Left is slightly smaller than the right due to the heart’s position Made up of highly vascularized tisse filled with alveoli May be visualized as a collection of millions of tiny bubbles surrounded with elastic connective tissue (stroma) Alveoli: Site of oxygen transfer to the blood Diaphragm: Muscular wall that separates the thoracic and abdominal cavities and creates negative pressure in the thoracic cavity to inflate the lungs Gastrointestinal System: Pharynx: Passageway from the mouth to the Esophagus Esophagus: Passageway from the pharynx to the stomach Stomach: Site of storage and mixing of food and adds digestive enzymes Small Intestine: Duodenum: First and most fixed portion of the small intestine as it leaves the stomach Jejunum: Next portion of the small intestine Ileum: Last portion of the small intestine and connects to the ascending colon portion of the large intestine Large Intestine Cecum: Does NOT connect the ileum and large intestine and is a diverticulum of the colon Colon: Connects to the rectum Rectum: Connects to the anal canal which exits the body Filtration and Excretory System 79 Eliminates waste products from the body Liver Largest gland in the body Produces bile Stored in the gall bladder Releases endocrine substances into the bloodstream to assist in the metabolism of fats and sugars Gall bladder: Stores and concentrates bile Pancreas: Secretes pancreatic juice for digestion, produces insulin in its islet cells, and has two lobes Kidney: Primary filter for blood and produces urine which is carried by ureters to the bladder Bladder: Stores urine prior to urination and excretes urine via the urethra Lymphatic System: Returns fluid and protein lost from blood in the capillary beds Lymph nodes, spleen, thymus, and the tonsils are phagocytic and extract foreign bodies Produce and circulate cells that are responsible for the bodies immune response Thymus: Located cranial-ventrally from the heart Spleen: Lays parallel to the greater curvature of the stomach Eye: Responsible for vision Ear: Responsible for hearing Male genital organs Scrotum: Skin pouch that encloses a testis Testes: Male gonads that produces spermatozoa Prostate gland: Accessory sex gland Penis: Composed of the roots, body, and glans Os penis: 10 cm long bone in the glans penis of the dog Female genital organs Ovaries: Paired organs that produce ova Uterine tube: Transports ova to the uterus Uterus: Conducts sperm to the uterine tube and consists of a neck and two horns Vagina: Dilatable canal from the uterus to the vulva Sensory Specific Organs Reproductive System: Part 7: Surgical Instruments The purpose of this module is to illustrate the most common instruments and give some recommendations for their use It should be noted that individual surgeons may use instruments in nonstandard ways 80 This is acceptable so long as the animal and procedure are not compromised Forceps Typically, forceps that require squeezing are used on tissue and those with finger rings and locking mechanisms are used on nonliving tissues Adson Forceps Have three teeth Useful for gripping tissue Adson-Brown Forceps Used for gripping tissue Less traumatic to tissue than non-toothed forceps Requires less crushing pressure to maintain a grip than non-toothed forceps which leads to less vascular damage Mayo (Russian) Forceps Useful for gripping delicate tissue Thumb forceps General purpose tissue gripping forceps Should not be used for “slippery” tissues since excessive gripping pressure will cause vascular damage Tuttle thoracic tissue forceps Used for gripping delicate and “slippery” tissues Ringtip distributes the pressure on the tissue Lack of teeth prevents punctures into vessels or lung tissue Singley tissue forceps Used similarly to Tuttle thoracic tissue forceps in the abdomen Prevents punctures into the GI tract Debakey thoracic forceps Large number of teeth distributes the pressure to help avoid punctures Cushing neuro tissue forceps Delicate tipped forceps for gripping AROUND nerves Not used for gripping the actual nerve Jewelers forceps Used for delicate gripping Does not grip tightly Babcock tissue forceps Used for atraumatic tissue holding of tissues that cannot be perforated, such as the intestines Allis tissue forceps Teeth tightly grip soft tissue Chester and Ballenger sponge forceps Hold gauze sponges for applications in body cavities Crille hemostatic forceps Have grooves running from tip to hinge This allows less slippage when tension is applied perpendicularly to the jaws Kelly hemostatic forceps 81 Have grooves running side to side of the jaws This allows less slippage when tension is applied parallel to the jaws Mosquito hemostatic forceps Similar to but with smaller jaws than Kelly forceps Useful for gripping small bleeding vessels Hartman mosquito hemostatic forceps Smaller versions of Mosquito forceps Mixter thoracic forceps Kelly-style forceps with a sharp angle towards the tip of the jaws Useful for gripping bleeding vessels at an angle Useful for positioning ligatures around vessels Collin gallbladder forceps Relatively atraumatic forceps used for retraction and gripping of the gall bladder Backhaus towel forceps Used for clamping sterile drapes or towels together when creating a sterile field May also be used to clamp sterile drapes directly to the skin Roeder towel forceps Have ball-stops to prevent towel slippage up the pins Edna towel forceps Grip towels without puncturing them due to flat pins Must not be used on skin due to crush damage Jones cross-action towel forceps A variation on Backhaus towel forceps Mayo dissecting scissors Used for dissection of tissue Often used for cutting sutures and sterile paper drapes Operating scissors Used for cutting suture and sterile paper drapes Metzenbaum dissecting scissors The primary scissors used for tissue dissection Also useful for cutting tissue Strabimus (“baby metzenbaums”) Used for fine dissection of tissue Stevens Tenotomy scissors Designed for cutting or dissection of a tendons and often used for fine dissection in other tissues Iris scissors Designed for fine dissection in the eye Often used for very fine dissection and creation of phlebotomies and arteriotomies Wire scissors Used for cutting orthopedic and approximation wire Towel Clamps Scissors 82 Lister bandage scissors Used for cutting bandage material Flared tip prevents catching tissue Weck Spencer suture scissors Have a cutout semicircle in the lower blade tip to allow it to slip under a tight suture Needle Holders Mayo Hegar needle holder Holds a suture needle for wound closure Olsen Hegar needle holder Contains scissor blades to allow easy suture cutting Finochietto and Debakey thoracic needle holders Have specialized tips and/or mechanisms to allow easier deep tissue suturing Castroviejo micro needle holder Used for delicate suturing and holding very small suture needles Gelpi retractors Used for holding open incisions for better visibility Weitlaner retractors Used for tissue retraction with a more distributed opening of that tissue than with Gelpi retractors Senn retractor Used for manual tissue retraction Bernay finger rake retractors Used for manual retraction of tissue Volkmann rake retractors Similar to Bernay retractors with a larger handle for improved handling Ochsner ribbon retractor (malleable) Highly bendable strip of metal that allows bending into whatever configuration will allow the best retraction Primarily used for abdominal retraction Parker retractors Nonflexible retractors Commonly used for abdominal retraction Deaver Retractors Used for abdominal retraction Mayo abdominal retractor Used for abdominal retraction O’Sullivan, O’Connor and Wexler abdominal ring retractors Used for holding multiple abdominal retraction paddles for handsfree retraction Love nerve retractor Used for holding individual nerves or fibers Balfour retractors Used for thoracic retraction Retractors 83 Finochietto rib spreader Used for spreading ribs for thoracic access Bailey rib approximator Used for bringing ribs together for closure following a thoracic procedure Cooley cardiovascular clamps Used for isolating sections of major vessels Partial clamping will isolate sections while allowing blood flow around the clamped section Renal clamps Used for clamping renal vessels Serrefines (bulldog) vascular clamp Used for clamping superficial vessels Yankauer suction tube Used for suctioning fluids out of the surgical field Debakey suction tube Another style of suction tip Periosteal elevators Used for retraction of the periosteum Lebsche sternum knife, mallet, Stille-Liston bone cutting forceps Used for cutting or splitting bone during orthopedic procedures Pilling-Ruskin, Beyer, and Jansen-Zaufel rongeurs Used for “chipping” off small pieces of bone Bruns bone and Spratt mastoid curettes Used for scraping and carving bone Emesis basin Designed for collecting vomit Often used for holding damp sterile sponges Sponge bowl Used for holding sponges and sterile fluids Silverman style Used for collecting tissue samples Used for absorption of fluids in a surgical field Clamps Suction Tubes Elevators Orthopedic Bowls Biopsy Needles Sponges Part 8: Sterilants, Disinfectants, and Antiseptics 84 Section 8.1: General Sterilization: The physical or chemical destruction of all microbial life including highly resistant bacterial spores Disinfection: The inactivation of most pathogenic organisms except for some highly resistant forms (spores) on inanimate surfaces and implies the destruction of the vegetative forms of bacteria but not spores Antiseptic: A substance that inhibits or destroys microorganisms on or in living tissue Asepsis: A state of freedom from infection Aseptic technique: The steps required to prevent contamination of the surgical site with infectious agents Methods of sterilization Filtration May be used for gasses or liquids Involves the separation of particulate matter of known sizes using a membrane Commonly used for pharmaceutical liquids Useful for heat sensitive media, offers high through-put, and provides absolute sterilization Unable to differentiate between similarly sized particles Radiation Usually gamma radiation from Cobalt60 Provides penetration through relatively impervious materials (i.e., metal and plastic) Leaves no chemical residue Can start a structural change in materials, especially some polymers, that may continue to develop over months Usually used by manufacturers due to cost of the process Thermal Most common form of surgical sterilization Believed to denature bacterial proteins Sterilization is a function of time and temperature Two types, dry and wet Dry heat (i.e. hotbead sterilizer) used on moisture sensitive materials such as oils, powders, and petroleum products Causes protein oxidation which requires longer exposure or higher temperatures Wet heat (boiling water and autoclave) kills bacteria via the coagulation of critical proteins Boiling water A poor sterilant at ambient pressure Has a relatively low temperature May be enhanced by the addition of sodium hydroxide or sodium carbonate 85 Destructive to instruments, especially glassware and rubber Autoclave Involves superheated steam under pressure to allow higher temperatures than normal steam can provide Requires materials and wrapping that are not damaged by heat, moisture, and pressure and are permeable to the steam. Requires complete saturation of the surgical pack for effective sterilization i.e., 121 degrees C for 13-15 minutes (5-10 minutes plus 3-8 minutes as a safety margin) Larger or denser packs require more time for adequate saturation with steam Time for the autoclave to reach temperature and saturate the pack is the heat-up time Prevacuum autoclaves achieve this heat-up time in 12 minutes Can be used for emergency “flash” autoclaving Also reach higher temperatures and so require shorter exposure times (131 degrees C for 3 minutes) Loads should be vented for ~10 minutes following a cycle to prevent condensation Liquid Chemical Important to differentiate between sterilants and disinfectants Sterilants are designed to kill all microorganisms, disinfectants are not Disinfectants are NOT adequate for instrument and implant/catheter sterilization Chemical sterilants are regulated by the FDA as medical devices EPA involved as well Approved chemical sterilants will say on the bottle that they are sterilants Nonsterilant chemicals will have disinfectant written on the bottle and should not be used for instrument or device sterilization Cold sterilants work by contact, care should be taken that all surfaces are in contact with the solution Flush sterilant through catheters, open all latches, etc. Cold sterilants are tissue irritants and require complete rinsing (sterile water or saline) of sterilized objects to remove all chemicals prior to tissue contact Aldehydes Glutaraldehyde Requires >12 hours of full immersion for sterilization of resistant spores Noncorrosive Activated solutions have less than 2 week shelf life Toxic to skin, eyes, respiratory tract Glutaraldehyde solutions come in different strengths Only those classed as a sterilant should be used for instrument or device sterilization Formalin 37% aqueous formaldehyde 86 Requires >24 hours of full immersion for sterilization of resistant spores Toxic to skin, eyes, respiratory tract Hydrogen Peroxide 6% aqueous solution with >30 minute exposure may kill some resistant spores in addition to less hardy microorganisms Potentially explosive at high concentrations Corrosive Irritant to skin and eyes Peracetic acid (35%) Steris System 1 Automated process suitable for endoscopic equipment Fast (3045 minutes) and has environmentally friendly byproducts Useful only for small loads of instruments and provides no sterile packaging, must be used immediately upon cycle completion Gas Chemical Requires an enclosed chamber similar to an autoclave Ethylene Oxide (ETO) Colorless gas Flammable, explosive, toxic, and irritating to skin and mucus membranes Destroys metabolic pathways by alkylation Typically requires 12 hours of exposure Time is inversely proportional to pressure Requires ~24 hours of ventilation after cycle Hydrogen Peroxide Safer than ETO to the user Requires little ventilation and shorter exposure times Cannot be used with absorbent materials such as paper or cloth Will condense into water and soak the packaging, preventing gas contact and allowing poststerilization “wicking” of microorganisms through the packaging Section 8.2: Sterilization Procedures All instruments must be thoroughly cleaned prior to sterilization Organic debris can dramatically extend the required time for sterilization i.e. Glutaraldehyde sterilization time increases from 12 hours to 48 hours in the presence of organic debris For most forms of sterilization instruments should be dry Not as important for steam autoclaving Some residual moisture is important for ETO sterilization Sterilization indicators should be used to verify proper sterilization of items Sterilization sleeves have indicators on them Packs should use indicators inside the inner wrap and under or inside any containers or large objects 87 Instruments should be open and accessible to steam or gas Open instruments allow steam or gas access to hinges and clasps Upside down bowl filled with gauze may not allow steam or gas to penetrate, leaving unsterilized items in the pack Items should be placed so as to not puncture the wraps or sleeves Packs and sleeves should be dated on the day that sterilization is completed Paper/plastic wrapped items/packs are typically sterile for 6 months when stored in closed cabinets Cloth wrapped items are considered sterile for 8 weeks when stored in closed cabinets Packs and sleeves that get wet or are punctured should be considered nonsterile If there is any doubt about sterility, consider it nonsterile Steps for Wrapping Packs Step 1: Lay Instruments on tray and place on two paper or cloth drapes Step 2: Fold corner of first drape tightly over the instruments, fold back to prevent overlap Step 3: Fold the side corners of the first drape tightly over the instruments, fold back to prevent overlap Step 4: Fold opposite corner of first drape over and tuck into the space created by the other three folds Step 5: Pack should be tightly wrapped with the first drape Step 6: Repeat steps 15 with the second drape, working from the opposite side Step 7: Tape the 2nd drape with indicator tape and label with contents and date of sterilization Section 8.3: Disinfection Primarily chemical High-level disinfectants used for instrument disinfection between rodent procedures, some oral procedures, and for delicate endoscopic equipment that cannot be sterilized Intermediate and some low-level disinfectants should be used for cleaning surfaces in surgery Disinfectants rated as high, intermediate, or low based on efficacy against microorganisms High-level Disinfectants Used for instrument disinfection between rodent procedures and for critical surface cleaning Aqueous Iodine Contains higher levels of free iodine Cytotoxic Stains surfaces 30 minutes of exposure for disinfection 88 Aldehydes 30-45 minutes of exposure for disinfection Sodium hypochlorite (bleach) Toxic Corrosive 3,000 ppm for 45-60 minutes for disinfection Phenol compounds (carbolic acid) No longer commonly used 30 minutes for disinfection Intermediate-level Disinfectants Used for cleaning surfaces Iodophor (Povidone iodine) Iodine complexed with surfactants or polymers for slower release of free iodine Dilution lowers cytotoxicity and increases bactericidal activity Rapidly deactivated in the presence of organic matter Residual activity of 46 hours May be mildly irritating Chlorhexidine Rapid onset and long residual activity (8-12 hours) Not deactivated by organic matter Nonirritating Considered superior to Iodophors due to residual action even when dried Low-level Disinfectants: Alcohols (Isopropyl) Bactericidal Ineffective against most spores and fungi Minimal residual effects and inhibited by organic debris Cytotoxic Degreaser 30 minutes exposure for surface decontamination Quaternary Ammonium (Quat, Zephiran) Bactericidal Work by dissolving outer coatings on some pathogens Some bacteria, including Staphylococcus aureus and Pseudomonas, have common resistant strains Ineffective against spores and some viruses May support growth of some types of bacteria Per CDC, is not an appropriate sterilant Low toxicity in stable solutions 10-30 minutes for surface decontamination Antiseptics: Commonly used for surgical site preparation on the patient and for surgeons to scrub hands and forearms prior to gowning and gloving 89 Intermediate level disinfectants are often acceptable antiseptics Principal antiseptics Chlorhexidine Considered more effective than the Iodophors Available as tinctures, solutions, and detergents Works by contact time, requires sufficient skin contact duration for effect Iodophors Available as tinctures, solutions, and detergents Works by contact time similar to chlorhexidine Principal antiseptics Alcohol Useful for low-level antisepsis Has minimal residual effects Evaporates rapidly and leaves no residue Inhibited by organic debris Not sufficient as the primary antiseptic per standard human and veterinary surgical texts Useful in conjunction with Iodophors or chlorhexidine Breaks up surface oils and surface tension Part 9: Principles of Aseptic Surgery Aseptic Technique: A series of techniques used in the conduct of surgery that prevent the contamination of the surgical site with infectious agents This is not an absolute as surgical procedures almost always introduce contaminants into the field The goal is to lower the concentration of microorganisms to below the level required for an infection to start Aseptic Technique Major bacteria involved in surgical wound infections are Staphylococcus aureus Streptococcus pneumoniae Pseudomonas multivorans (not a bacteria) Coagulasenegative Staphylococci Enterococcus spp Escherichia coli In general, bacteria do not directly affect tissue cells Bacterial waste products such as endotoxins and cytotoxins damage and kill cells The body provides bacteria with food, shelter, humidity, and warmth Generally, bacteria are localized until they gain entrance to the bloodstream, then they become systemic infections The body fights extracellular bacteria and other organisms in an antibody mediated immune response Briefly, antibodies recognize foreign bodies and call in phagocytes 90 These phagocytes invaginate (envelope) and kill the bacteria, dying in the process Immune response causes an increase in temperature, production of pus, and influx of serous fluid Surgical infections retard healing by: Damage from toxins Physical debris Changes in local circulation Patient Surgical preparation Clean the site if needed Some animals, especially livestock, may have large amounts of contaminants (feces, dirt, bedding material) on their fur/skin These contaminants may be removed prior to surgery with bathing or washing Be aware that getting the animal wet may reduce their temperature, especially if already anesthetized Bathing is best performed the day prior to surgery Surgical preparation Remove excess hair Hair should be clipped close to the skin around any potential incision sites. An area should be clipped around this incision sufficient to create a surgical field In rodents, this should be a minimum of 13 cm from the incision site In larger animals, this should be a minimum of 46 cm from the incision site Shaving with a blade is not generally recommended as skin trauma may lead to increased bacterial counts Clipping hair the day before surgery is associated with higher bacterial counts due to skin trauma and should generally be avoided All hair should be removed from the animal and prep site with a vacuum For rodents, sticky tape may be used to remove hair instead of using a vacuum by pressing the tape to the clipped hair It is recommended that gloves be worn for the following steps to minimize contamination from the technician’s hands Correct attire should also be worn (discussed later) Surgical preparation Surgical scrub on the skin A typical scrub consists of an application of Chlorhexidine or povidone iodine scrub soaked gauze sponges followed with a wipe with 70% Isopropyl alcohol or sterile water soaked gauze sponges With oily or dirty skin, an initial wipe with 70% Isopropyl alcohol may be performed to remove oils and reduce surface tension Remember that the evaporation of alcohol may cause a significant loss of body heat, particularly in rodents and neonates May be reduced by heating the containers of prep solution in a 100-105 degree F hot water bath Alcohol soaked gauze sponges should be damp but not dripping wet 91 Warm sterile water may also be used This scrub should be performed 3-5 times Each scrub should begin over the incision site and follow a concentric circle away from the incision site The same gauze sponge should never return to the incision site or a previously cleaned area When surgically preparing a limb, it may be useful to tape the foot to an IV stand and suspend it for prepping Start at the highest point and continue the concentric circles downwards (with gravity) When the final scrub has dried, apply povidone iodine or chlorhexidine solution to the surgical site Commonly the final 12 cycles of the surgical scrub and application of an antiseptic solution is performed after the animal is moved to the operating room (OR) and placed in proper recumbency A povidone iodine film (i.e., 3M DuraPrep) may be applied instead of the antiseptic solution Allow the antiseptic solution or film to dry prior to draping Surgical Mindset If there is a question as to whether a step has been performed incorrectly, assume that it has and start over If there is suspicion that a prepped surgical site may have become contaminated, redo the entire prep If there is suspicion that a drape in the operating field has become contaminated by contact with a non-sterile material replace it or, if this is impractical, cover the suspected contaminated area with a new sterile drape Surgical Positioning The surgeon(s) should direct how the animal is positioned on the table Surgeon’s often have positioning “quirks” which allow them to better visualize the surgical site Positioning is named by the part of the body in contact with the table Right lateral recumbency = animal laying on its right side Dorsal recumbency = animal laying on its back Sternal or ventral recumbency = animal laying on its sternum The animal may be kept in place with the aid of sandbags, troughs, rolled towels, vacuum positioning “beanbags”, limb ties, or incisor clips (for rodents) Care should be taken that limb ties do not occlude normal blood flow For procedures where skull stability is important the animal may be placed in a stereotaxic device Note, care must be taken to ensure that the clamps and earbars are not too tight and cause skin or eardrum trauma The Sterile Field When the animal is properly positioned and antiseptically prepped for surgery, a sterile field should be created 92 Generally, a sterile field should be large enough to prevent accidental contamination of the incision site(s), operating team, and sterile instruments and equipment Rodents At a minimum, a sterile fenestrated drape whose opening only uncovers the surgical site is recommended Commonly the drape covers the entire animal A paper drape can make it difficult to monitor respiratory and cardiovascular functions during surgery A clear plastic “Ziplock” bag can be cut into a flat sheet and gas sterilized for use instead Allows easy viewing of the animal Clear plastic adhesive surgical drapes may also be used A table drape may also be placed prior to positioning the animal on the table to enlarge the field Non-rodents At a minimum, a sterile fenestrated drape sufficient to cover the animal and surgical field is recommended Sterile cloth or paper drapes may be used and should cover all areas except those immediately about the incision site(s) Towel clamps may be used to affix the drape(s) in place Alternatively, sterile surgical staples may be used A sterile plastic adhesive incise drape (i.e., 3M Steridrape) may be placed over the drape fenestration Extends the field to the edge of the incision and limits possible contamination from exposed skin Can be difficult to remove from instruments and equipment For rodents, sterile bandaging patches (i.e., Teguderm) are more conveniently sized and may be used instead Equipment such as drill cables, Carms, and imaging equipment that cannot be sterilized and will be in the surgical field should be draped or covered in sterile sleeves It is convenient to sterilize light handles or provide sterile covers so that sterile personnel can adjust operating lights during surgery Special Cses Draping limbs Easier on a suspended limb A surgeon may handle the unbandaged portion of the limb using a sterile towel or drape Alternatively, an extra pair of sterile gloves may be worn and discarded after draping These gloves should not come into contact with the drape or instruments as they are no longer sterile The suspending tape should be cut close to the limb and a sterile stockinette placed on the foot and unrolled towards the body 93 The wrapped limb may be passed through a hole in the sterile drape When the incision is made, the stockinette should be affixed about the incision with towel clamps or staples Alternatively, the limb may be completely wrapped in sterile adhesive drapes Stereotaxic positioning Difficult to maintain sterility as the horizontal slides must be accessible for movement of the manipulator arms yet must be used during positioning of the animal prior to creation of the sterile field Option 1: Drape over the animal and the stereotax Cut the drape over the slide(s) to be used Discard scissors Push the drape down under the slide Place the sterile manipulator arm on the slide Place small drapes over the slide Option 2: Cover the slide(s) to be used with a double cover of stockinette Cut small holes to allow the ear bar clamps to protrude through the stockinette Sterilize the slide frame assembly Place the animal in the stereotax and prep Cut the first stockinette away with sterile scissors and discard both stockinette and scissors Drape over the animal and stereotax Cut the drape over the slide and the second stockinette Clamp the stockinette to the drape and place the manipulator arm on the slide Surgical Personnel Attire and operating personnel preparation Street clothing, especially shoes, is a major source of contaminants Street clothing should be replaced with clean scrub suits Recommended to tuck scrub tops into pants to reduce the dispersion of skin debris During hair clipping and prep a clean labcoat or Tyvek coverall can be worn to protect the scrubs Street shoes should be replaced with sanitized OR-specific shoes or covered with shoe covers Safety glasses are recommended and are mandatory for nonhuman primate surgery Surgical caps should be worn to prevent contamination from shedding hair Facial hair should also be covered Surgical masks should be worn to filter expired air Masks are short-term filters only and should be replaced between surgical procedures 94 Exam gloves are helpful to minimize skin contaminants Long-term exposure to antiseptic and cleaning solutions can be irritating to the skin as well Scrub with an antiseptic soap (chlorhexidine, povidone iodine) Antiseptic solutions work by contact over time Scrub should take ~10 minutes (~5 minutes per cycle) to allow sufficient time for the antiseptic scrub to work Hands should be kept higher than elbows during the entire process to maintain drainage from hands down to elbows Once the scrub is begun, hands and forearms should only touch sterile surfaces If there are any breaks in technique, start over Steps for a surgical scrub and donning a surgical gown Wet hands and forearms Apply antiseptic scrub to both hands and forearms Scrub hands first, followed by forearms Perform one cycle, rinse, repeat cycle, rinse Anatomical (column) scrubs: scrub each skin surface (four sides of fingers, top of hand, etc.), ten to fifteen times Timed scrubs: scrub each surface for a set amount of time (~5 minutes per cycle) Some research suggests that a final covering with the antiseptic soap prior to toweling dry enhances antimicrobial activity during surgery Dry hands with a sterile towel Use opposite corners for each hand so that if one hand is unknowingly contaminated the contaminants are not transferred to the other hand Keep hands and forearms well away from scrubs and any nonsterile surfaces Hands are not sterile and should be considered to contaminate any sterile objects that they touch Don a sterile gown Gowns should only be handled by inside surfaces so as to not contaminate the outside of the gown Gowns may be picked up by the collar or the inside shoulder seams If required, gently shake the gown to allow it to unfold completely Slip arms into sleeves Do not allow hands to protrude from the cuffs Sterile operating personnel For batch surgeries of rodents, sterile gowns may be considered too expensive or cumbersome Consider wearing the same sterile gown for multiple surgeries or wearing sterile Tyvek sleeves Requires greater attention to possible instrument or device contamination by touching nonsterile skin or scrubs Don sterile gloves 95 Closed gloving is recommended as it minimizes chances for contamination Open gloving may be used for changing gloves but has a much greater risk of contamination If a sterile assistant is available, it is preferable to have them put the glove on the surgeon rather than open glove Open gloving is useful when performing multiple rodent surgeries Rinse gloves free of powder after donning Closed Gloving Step 1: Lay the glove on the cuff above the palm of the hand with the thumb side down and fingers towards the elbow Grasp the palm side of the glove through the cuff Step 2: Grasp the top side of the glove with the other hand through the cuff Step 3: Pull the cuff of the glove over the cuff of the gown Advance fingers past the cuff and into the glove Step 4: Pull the glove cuff as far down the sleeve as possible If performed properly, the cuff of the gown should cover the back of the hand to the fingers under the glove Step 5: Repeat steps 1-4 with the other hand Open Gloving Step 1: Extend hands from sleeves Contamination risk may be lessened by keeping the fingers of the assisting hand below the gown cuff Step 2: Grasp the inside of the glove’s cuff with the assisting hand Step 3: Pull glove over the hand and gown sleeve Do not unroll the glove cuff Step 4: Slip the gloved fingers beneath the cuff of the other glove Step 5: Slide hand into new glove and pull over hand and sleeve Step 6: Taking care not to touch contaminated surfaces, unroll the glove cuffs over the gown sleeves Adjust the glove Behavior in the operating room Unnecessary movement disturbs air currents and can increase airborne contaminants Non-sterile objects such as equipment and non-sterilely gloved hands should not be passed over sterile fields The number of personnel in the OR should be kept to a minimum Non-sterile personnel should keep clear of sterile areas All personnel should always be watching for and bringing attention to any compromise in sterility/aseptic technique Attention should always be paid to the maintenance of a sterile field Consider everything below the level of the table non-sterile Gown portions, equipment cables, suction hoses, dropped instruments, etc. 96 Backs of gowns are also considered non-sterile due to the difficulty in paying attention to them When not using them, keep hands above waist level Clasping the hands reduces the chance of inadvertently brushing them against a non-sterile object Attention should always be paid to the maintenance of a sterile field When passing other sterile personnel, pass back-to-back Instruments which purposefully (i.e, scissors cutting drapes and coming into contact with the contaminated underside) or accidentally (i.e, dropped off the sterile field) become contaminated should be immediately discarded from the sterile field Take all supplies in a sterile manner Surgical packs: Nonsterile personnel should open the outer wrap, taking care not to contaminate the inside of the outer wrap or the inner wrap Sterile personnel should open the inner wrap and either use the inner wrap as part of the sterile field or remove the instruments from the pack Surgical sleeves or foil packs Nonsterile personnel should open these Sleeves and foil packs should not be opened directly over the sterile field, sterile personnel can remove the item from the sleeve or the nonsterile person can flip it onto the sterile field Sterile fluids When opened, fluid should be poured from the bottle into a waste basket to flush possible contaminants from the bottle mouth Sterile personnel can pass the bowl under the stream until the bowl is filled Fluid should continue being poured until the bowl is removed from the stream; this prevents draining of fluid along the nonsterile outer surface of the bottle into the sterile bowl Fluids can be taken from a bottle or bag with a septum with a needle and syringe Wipe the septum clean with a 70% Isopropyl alcohol wipe A non-sterile person should hold the bottle or bag at a downward angle The sterile person pushes the needle through the septum and aspirates fluid The needle is potentially contaminated and should be discarded Surgical techniques If there is a break in technique If the drape becomes contaminated, cover with a fresh drape (adhesive drapes work well for this) If a glove or gown gets punctured, replace it immediately; evaluate any instruments that might have been handled for contamination If the incision site becomes contaminated, remove visible contaminants and lavage with large quantities of sterile isotonic fluid 97 Subsequent irrigation with povidone iodine or chlorhexidine solution may be helpful In any of these cases or when a break is discovered after the procedure is completed, antibiotics may be used after consultation with a veterinarian Pay careful attention to the animal for 23 weeks postsurgery and observe for any signs of infection such as erythema, swelling, signs of pain and/or discomfort, abnormal discharge, and loss of function Rodent Surgical techniques Rodents such as mice and rats do get infected Rats are the most common model for infection in antibiotic testing Small incisions, quick surgeries, and a good immune system coupled with a fast metabolism may allow less stringent surgical conditions Attention to aseptic technique is still required Sterile drapes and surgical preps are recommended Sterile gloves are recommended Sterile gowns or sleeves may be useful Part 10: Endoscopic Procedures Section 10.1: General Endoscopy: “to look inside” Usually reserved for the interior of hollow viscera (bronchi and intestinal tract) Can be applied to laparoscopy and thoracoscopy as well Also known as Minimally Invasive Surgery (MIS) Surgical techniques designed to minimize the anatomic approach to the target surgical site Requires extensive equipment, training, and practice Requires training not only for the operating personnel but also for the preoperative and especially anesthesiology personnel People must know their jobs and perform them properly without supervision Require multiple steps and procedures Packs should be easily available if the need arises to transform to an open procedure Room layout must be planned beforehand and orientated towards a smooth operating flow and lack of obstruction Surgeon(s) and camera operator needs easy viewing of the monitor Section 10.2: Endoscopic Equipment Video-imaging equipment Can be either flexible or rigid Flexible endoscopes are usually used for GI and examination of the respiratory system 98 Rigid endoscopes (telescopes) are more commonly used Provide the most light Provide the largest viewing field Greatest resolution and clarity Larger scopes provide more light and better fields of view 10 mm laparoscopes are preferred for most procedures <5 mm laparoscopes are ideal for diagnostic laparascopy and thoracoscopy Operating endoscopes are rigid scopes with channels for inserting instruments such as biopsy collectors Viewing fields may be straight (0 degrees) or at angles Field of Vision: the borders of the field of view The closer the tip of the endoscope is to the target tissue the greater the magnification Camera operator may need to adjust the focus Endoscope must be attached to a camera, camera control unit, and monitor Actually, the endoscope can be looked down like a telescope but this is poorly suited for much beyond biopsies Lights Older systems use tungsten bulbs Newer systems use mercury, xenon, or halogen bulbs and are significantly brighter. Brightness needs to be adjustable to prevent washing out the image Are transmitted by fiberoptic cable This bundle of cable should be clean at both ends and replaced when enough (commonly 20%) of the cable break and fail Camera Use either one or three chips to convert the camera’s image into an electronic signal to be sent to the monitor Single-chip offers 450 lines of resolution Three-chip uses a separate chip for red, green, and blue and offers 600 to 700 lines of resolution Better systems avoid “whiteout” from glare reflected off light colored tissues Camera Control Unit: Allows adjustment of the camera images and transmits signal to the monitor Can adjust focus, sharpness, etc. Monitor Offer resolution between 400 and 700 lines of resolution Should be optimized for the camera chips used Must be medical grade and properly grounded May be ported to VHS systems for recording Insufflator Abdominal cavities are commonly filled with an inert gas (carbon dioxide or nitrogen) to prevent collapse of the cavity and difficulty with surgery Insufflator provides constant pressure in the cavity 99 May be electronic or mechanical Electrosurgery Used for hemostasis and cutting Ultrasonic or laser devices may also be used Irrigation and Suction Used to keep the surgical field clean and allow easy vision Commonly combined into one device Insufflation needles Used to create a sealed breach into the abdominal cavity to administer gas May be disposable or reusable Trocars Used to create “ports” for the entry of instruments and materials into the body cavity Is an obturator enclosed in a sleeve Ports A device that holds open an incision to allow passage of endoscopic instruments into a body cavity Ports may be placed by an open technique rather than using trocars More time consuming Useful when adhesions may be present or when trying to avoid organs such as the rumen Reducers Act as additional gaskets inside or on top of the trocar for the maintenance of pneumoperitoneum Endoscopic surgical instruments are used and look identical to standard instruments at the working end The instruments are connected to a shaft which is passed through the trocar or port Section 10.3: Endoscopic Anesthesia Differs from standard anesthesia due to the increased cavity pressure caused by insufflation Thoracic procedures usually do not use as the collapse of one lung and the rib cage allow an open surgical site Insufflation increases the abdominal pressure and pushes the diaphragm into the chest cavity Increased intraabdominal pressure (IAP) increases intrathoracic pressure and thus requires mechanical ventilation at higher pressures than normal PaCO2 Arterial carbon dioxide increases when the insufflation gas is CO2 by transperitoneal absorption If PaCO2 goes up, PaO2 goes down to compensate This results in less O2 being free in blood 100 Increase in heart rate Increase in mean arterial pressure (MAP) Increase vascular resistance Decrease cardiac output Decreases arterial and visceral blood flow Positioning in Trendelberg (head down) or Fowler (head up) also shift pressures in the abdomen and chest Insufflation For some procedures, an increased working space is needed in the chest and inert gasses are introduced to expand the cavity This can be dangerous as it limits lung capacity Requires relatively little increases (5 mmHg) to clear space EtCO2, MAP, heart rate, and CO will increase Requires single lung ventilation Standard endotracheal tubes inflate both lung equally Defeats the purpose of insufflation Single lung respiration requires specialized endotracheal tubes The inferior lung receives 60% of the C.O. Hypoxic pulmonary vasoconstriction (HPV) diverts blood flow from an atelectatic lung Inhalation anesthetics inhibit HPV, injectable barbituates commonly do not Due to a constant and maintained pressure, liquids or inert gasses may by infused between tissues to dissect them apart