Guided light and diffraction model of human
advertisement

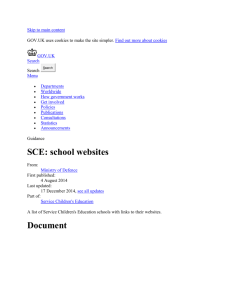
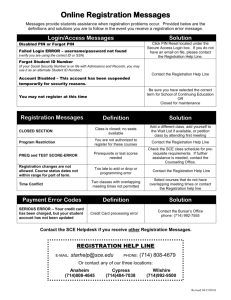
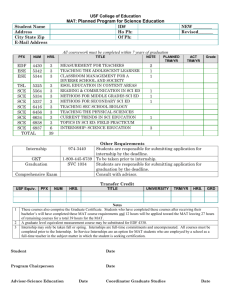
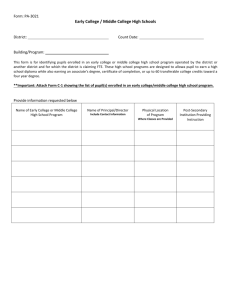
2318 J. Opt. Soc. Am. A / Vol. 22, No. 11 / November 2005 Vohnsen et al. Guided light and diffraction model of human-eye photoreceptors Brian Vohnsen, Ignacio Iglesias, and Pablo Artal Laboratorio de Óptica, Universidad de Murcia, Campus de Espinardo (Edificio C), 30071 Murcia, Spain Received November 8, 2004 The photoreceptors of the living human eye are known to exhibit waveguide-characteristic features. This is evidenced by the Stiles–Crawford effect observed for light incident near the pupil rim, and by the directional component of light reflected off the retina in the related optical Stiles–Crawford effect. We describe a model for the coupling of light to/from photoreceptors on the basis of waveguide theory that includes diffraction between the eye pupil and the photoreceptor apertures, and we show that valuable insight can be gained from a Gaussian approximation to the mode field. We apply this knowledge to a detailed study of the relationship between the Stiles–Crawford effect and its optical counterpart. © 2005 Optical Society of America OCIS codes: 170.4470, 330.4060, 330.5310. 1. INTRODUCTION The human eye has long been known to be sensitive to the angle of incidence of light on the retina as demonstrated by the psychophysical Stiles–Crawford effect (SCE), i.e., a reduced visual sensitivity to light rays that intersect the eye pupil off axis1 with a resulting impact on visual performance.2 The directionality also manifests itself in light reflected off the retina3 in what is often referred to as the optical Stiles–Crawford effect (OSCE).4 In either case the responsible mechanism has been identified with the waveguide nature of the photoreceptors.5–8 In an unaberrated eye the photoreceptors are oriented with their central axis aimed toward a point near the pupil center9 since this is the configuration where their coupling to incident light is favored the most. Centered at the same pupil point, and superimposed on an essentially uniform background of scattered light, the OSCE produces a peaked intensity distribution of directional light returned from the eye fundus.10–12 Both the SCE and the OSCE are normally fitted to a Gaussian function at the pupil plane (for simplicity assumed to be rotationally symmetric although its actual cross section might be slightly elliptical13): f共r = 冑x2 + y2兲 = C + A ⫻ 10−r = C + A exp共− ␣r2兲, 2 共1兲 where C is a constant background, A / 共A + C兲 determines the degree of directionality, and the parameters and ␣ = / log共e兲 ⬇ 2.30 both represent the spread in directionality (a smaller value equals a larger spread). Although they are similar in origin, there is an important distinction between the intensity distribution measured for the OSCE and the sensitivity curve measured for the SCE. The directionality parameter, as measured with both methods at approximately the same retinal location, differ typically by a factor of 2, with the OSCE distribution being the narrowest.10,12 With simultaneous scanning of both the entrance and the exit pupil of the eye, even higher values for the OSCE directionality parameter 1084-7529/05/112318-11/$15.00 (equaling 3 to 4 times the value for the SCE) have been found.4,14 It has been suggested that both the SCE and the OSCE are caused by variations in photoreceptor alignment,13,15 but this contradicts the finding of highly aligned foveal receptors.16 Moreover, it has been argued that the observed narrowing of the OSCE, as compared with the broader acceptance curve for the SCE, could be caused by an energy redistribution among modes in the photoreceptors following reflection.14 Recent studies have favored a model that explains the directionality through a combined effect of photoreceptor wave-guiding by light-ray excitation and a scattering mechanism from the simultaneous illumination of many photoreceptors.17,18 Both may produce a Gaussian peak at the pupil and the combined effect of two distributions can explain the observed radiation narrowing for the OSCE. Another concern has been the dependence on wavelength for both effects.19–21 Experimental studies of the OSCE21 have verified a 1 / 2 dependence in agreement with the photoreceptor scattering model.17,18 In this case the contribution from wave-guided light is assumed to be wavelength independent so that scattering alone produces the dependence observed. Other studies of the SCE have suggested a more delicate wavelength dependence to take into account also minor variations produced as the number of allowed photoreceptor modes change.7 In this paper we propose an alternative model that can account for both the known deviation between the directionality parameter of the SCE and the OSCE and the observed overall 1 / 2 dependence. Instead of considering that light propagates as rays in the eye to/from photoreceptors as commonly assumed, the model includes diffraction of both the incoming beam and the light emanated by the photoreceptors. This choice is dictated by the small size of the photoreceptor apertures and is supported by both the observation of individual modes6 and current models of light propagation inside photoreceptors.7,14,22,23 © 2005 Optical Society of America Vohnsen et al. Our description is applicable on a single-photoreceptor level and therefore appears highly suited also for analysis of directional light in the context of imaging with a confocal scanning laser ophthalmoscope.24 The paper is organized as follows. In Section 2 the model that accounts for the coupling of light to an individual photoreceptor and the corresponding generation of a back-reflected directional component is described. The importance of the model for the SCE is discussed in Section 3, and the relation to the OSCE is discussed in Section 4. In Section 5 the influence of a Maxwellian illumination scheme is addressed for both effects, and in Section 6 the influence of coherence is discussed. In Section 7 we present our conclusions. Vol. 22, No. 11 / November 2005 / J. Opt. Soc. Am. A at which the intensity has dropped to 1 / e2 ⬇ 0.14 of the on-axis value) is incident on the eye and focused on the retina to a spot size of wr = In the following the model of light coupling to and from photoreceptors, including diffraction of both the incoming beam towards the retina and the back-reflected light, is described in accordance with the notation shown in Fig. 1. A collimated Gaussian beam of width 2w0 (i.e., the width Pr 共2兲 neyew0 = 冏冕冕 * r m dxdy 冏 2 共3兲 . Here r is the normalized incoming field amplitude at the retina and m is the normalized mode field amplitude (both including their phases), and the integration is to be carried out in the retinal plane.25 Ideally, with only one photoreceptor the area of normalization and integration in Eq. (3) extends to infinity. This methodology will be used in the following derivations. However, for a wellconfined mode and illumination, the area to be considered needs to be only slightly larger than the actual photoreceptor aperture. This approximation will be used in the numerical work of Section 5 (see also footnote of Table 2 below). The total power carried forward by the photoreceptor is distributed among a small number of modes,6,7 but when the incident beam is Gaussian and wellmatched to the location and width of the photoreceptor, the largest coupling will be to the fundamental mode LP01, given that for a cylindrical waveguide this mode is nearly Gaussian.26 When this is not the case more of the incident field may couple to higher-order modes. The actual number of possible modes is found from the V number of the waveguide defined by V = 共di / 兲冑ni2 − n2s , where the (assumed) uniform inner segment has refractive index ni ⬇ 1.353 and width di, and the refractive index of the surrounding medium is ns ⬇ 1.340 (the values shown are those used by Snyder and Pask; see Ref. 7 and references therein). When V ⬍ V0 = 2.405 only the fundamental mode is allowed which for the selected parameters occurs if di ⬍ 4.09. The fraction of light that remains uncoupled is essentially lost from the photoreceptor but might be absorbed while traversing adjacent receptors.28 For the present, we assume that the incident beam couples light only to the fundamental mode represented by a Gaussian function of width 2wm. In this case the fraction of power transmitted to the photoreceptor T = Pm / Pr can be found from Eq. (3) as27 T共u兲 = Fig. 1. Schematic of the configuration considered for light coupling to and from a photoreceptor. (a) The Gaussian beam incident on the eye is focused at the retina slightly off axis from the photoreceptor axis and coupled to mode m. (b) The incident Gaussian beam is displaced in the pupil a distance rp with the result of intersecting the photoreceptor at an angle . (c) The back-reflected beam diffracts from the photoreceptor and produces a Gaussian intensity distribution at the eye pupil. feye in the absence of aberrations. The eye parameters used are those of the reduced eye, i.e., a focal length feye = 22.2 mm and a constant index of refraction neye = 1.33. The focused beam can couple its power Pr at the retina to the guided modes of an underlying photoreceptor, where the amount of power Pm coupled to mode m can be expressed via an overlap integral as Pm 2. MODEL OF LIGHT COUPLING TO AND FROM PHOTORECEPTORS 2319 冋 2wrwm 2 w2r + wm 册 冋 2 exp − 2u2 2 w2r + wm 册 , 共4兲 where u is the displacement of the incident beam with respect to the photoreceptor axis [Fig. 1(a)]. Alternatively the incident beam may intersect the photoreceptor at an angle = rp / feye Ⰶ 1, where rp is the shift of the incident beam in the pupil plane with respect to the central posi- 2320 J. Opt. Soc. Am. A / Vol. 22, No. 11 / November 2005 Vohnsen et al. tion [Fig. 1(b)], with the fraction of transmitted power given by27 T共兲 = 冋 2wrwm 2 w2r + wm 册 冋 2 exp − 2共neyewrwm兲22 2 2共w2r + wm 兲 册 . 共5兲 In the case in which the beam is both shifted and tilted with respect to the photoreceptor axis, the transmission factor can be found to a first approximation by a combined action of Eq. (4) and the exponential factor from Eq. (5). When the width of the incident beam is perfectly matched to that of the mode, wr = wm = w, these expressions reduce to 冋 册 w2 共6兲 , 冋冉 冊 册 T共兲 = exp − neyew feye neyewm 共9兲 . In the following sections we apply this photoreceptorto-light coupling model on both the SCE and the OSCE. The description focuses on cone photoreceptors, as this is where most experimental data are available, but the principle ideas should be equally applicable to rods albeit the directionality, and thus the wave-guiding effect, manifests itself less directly for them than it does for cones.6,31,32 3. STILES–CRAWFORD EFFECT REVISITED − u2 T共u兲 = exp wp = 2 2 . 共7兲 In the above derivations the refractive index of the inner photoreceptor segment, which is slightly larger than that of the reduced eye, enters indirectly through the calculation of the mode width 2wm. In turn, the small reflective loss 共⬃0.01% 兲 due to the difference in indices has been omitted as it is clearly negligible when compared with other factors. It is known that in order to avoid absorption of visible light in the outer photoreceptor segment, and thereby enhance the contribution of directional back-reflected light to the OSCE, bleaching of the photoreceptors is required. Thus, the directional light originates either from reflection at the outer photoreceptor end face or beyond,12 or possibly from the refractive index modulation by the numerous photopigment-containing discs in the outer segment itself.29 In any case only a small number of scattering processes can be involved since the directional component retains a large degree of the incident light polarization (although the exact amount, apart from being wavelength dependent, is still a topic of debate).10,30 The scattering will excite one or more back-reflected modes, but for simplicity we again assume that only the fundamental Gaussian-like mode is efficiently excited. As this field radiates from the photoreceptor it diffracts in the eye and ideally produces a Gaussian beam largely centered at the eye pupil. This beam generated by the photoreceptor’s light guiding carries forward an intensity distribution [Fig. 1(c)] Idir共r兲 = I0TR exp关− 2共r/wp兲2兴, 共8兲 where I0 is a constant (for a given incident power and spot size), T is the incident coupling efficiency as determined by Eqs. (4)–(7), and R is the (directional) photoreceptor reflectivity. It should be stressed that only the fraction of scattered light coupled back through the photoreceptor is contained in R. The unguided components are either lost (absorbed) or contribute to a practically uniform background at the pupil [not included in Eq. (8)].10 The spot size at the pupil, wp, is given by [cf. Eq. (2)] The width of the sensitivity curve for the SCE is traditionally obtained by registering changes in apparent brightness while translating an incident beam in the pupil plane and fitting the results to Eq. (1). The translation produces a corresponding change in angle of incidence at the retina and the coupling efficiency is therefore described by T共兲. When the beam is well matched to the fundamental mode of the photoreceptor, the directionality parameter SCE can be obtained directly from Eq. (7) as SCE = log共e兲 冉 冊 neyew feye 2 . 共10兲 A similar analysis has previously been carried out for the case of an incident plane wave.7,14,33 Experiments with green, yellowish, and red light have shown that, some variation apart, SCE ⬇ 0.05/ mm2 at the fovea,1,4,7,12,13 which may be restated by use of Eq. (10) as w / ⬇ 1.8 (or w ⬃ 1.1 m). It should be stressed, however, that these experiments have been performed with simultaneous illumination of many photoreceptors and may therefore differ from the above-expressed result. We return to this point in Sections 5 and 6. The wavelength dependence of the SCE19 reveals an influence that has been related to the number of excited modes7 and to the spectral properties of the eye constituents,29,34 but for wavelengths either in the blue–green range or for utmost red the major trend fits the 1 / 2 dependence shown. To get beyond this tendency in a satisfactory manner would require a unification of the waveguide model with absorption in the entire eye, which lies outside the scope of the present study. However, it should be stressed that only with a sufficiently dim light source and a dark-adapted eye can the waveguide contribution to the SCE be properly accessed, since in this case the incident light will be effectively absorbed within a fraction of the entire outer segment (thereby eliminating the possible contribution of backscattered light34 to the sensation of brightness). The observed increase in SCE with distance from the fovea35 (approximately doubled at 2 deg eccentricity and beyond36) may also be explained by Eq. (10) since w increases with the waveguide width and thus with foveal distance. Finally, the fact that rods appear less directionally sensitive31 might be explained by a smaller mode width as compared with that of cones (due to their smaller size and possibly smaller index of refraction7). Vohnsen et al. Vol. 22, No. 11 / November 2005 / J. Opt. Soc. Am. A 4. OPTICAL STILES–CRAWFORD EFFECT REVISITED As the excited mode field propagates in the outer segment or beyond, scattering excites the backward-propagating modes that when diffracted combine to produce a Gaussian peak of directional light at the pupil. As the outer segment is very narrow, do ⬃ 1 m, it appears reasonable to assume that principally the fundamental mode can be efficiently excited by back-scattered light, as also suggested by simulations (see Table 2),7,14 with the field matched to the same mode of the inner segment.37 It should be stressed that this alone may suffice to explain the Gaussian distribution of directional light at the pupil. Actually, assuming a (uniform) refractive index7 no ⬇ 1.430 and estimating the corresponding V number one finds that for ⬎ 0.652 m only single-mode propagation is allowed in the outer photoreceptor segment. Several mechanisms have been proposed to explain the observed deviation between the widths of the OSCE and the corresponding SCE sensitivity curve, and a number of different but related methods have been used in part to resolve this controversy.4,8,10–12,14,17,18,20,21 The use of different techniques and illumination wavelengths, however, makes an exact comparison of the results obtained difficult. The OSCE has typically been analyzed by aligning the incident beam at or near the pupil center where the SCE is maximized, and subsequently recording the reflected light intensity versus pupil coordinate as this configuration carries the strongest imprints of directionality.10–12,20,21 This differs from the technique commonly used to analyze the SCE where the incident beam is translated in the pupil plane (Section 3). Thus, for the OSCE with a fixed but optimal angle of incidence we may take T共兲 = 1 and from Eqs. (8) and (9) directly obtain OSCE = log共e兲 2 wp2 = 2SCE , 共11兲 in good agreement with experimental studies of both effects when measured on the same subjects.12 A significantly larger value has been found in a similar study where only a narrow slit defines the exit pupil,20,21 but in that case the deviation is presumably due to the illumination of a larger retinal area with increased contributions from wider cone photoreceptors. As a numerical example we may take directionality parameters OSCE ⬇ 0.11/ mm2 (at the fovea) and OSCE ⬇ 0.19/ mm2 (at 2 deg eccentricity) measured at a wavelength = 0.543 m by Marcos and Burns (see Ref. 18). When inserting Eq. (9) into Eq. (11) these values may be converted to corresponding mode widths of wm ⬇ 1.03 m and wm ⬇ 1.35 m, respectively. If the inner segment potentially allows for the propagation of several modes (i.e., assuming that V Ⰷ V0) its width may be estimated by di ⬇ V0wm. This relation has been obtained by fitting a Gaussian distribution to the exact mode shape (see Ref. 26 in the limit of U01 → V0. For a waveguide with a given V an optimum fit to the Gaussian mode should be made numerically, but a good approximation is obtained by wm ⬇ di / U01). Thus, in this approximation one finds a diameter di ⬇ 2.5 m for the inner segment at the fovea in good agreement with 2321 anatomical studies whereas an estimated diameter di ⬇ 3.2 m at the eccentric location is somewhat smaller than expected 共⬃4.5 m兲.38 The reason for the deviation at the off-center location may be an increased contribution from higher-order modes like LP11, possibly excited by intrareceptor scattering of back-reflected light. If this is the case, the diameter is expected to fall within the range V0wm ⬍ di ⬍ 1.593 V0wm. It should also be recalled, however, that there are variations among subjects and that the parameters used above represent an average of only three. The method for the OSCE can be reconfigured to access the SCE in a more direct manner by measuring the reflected light distribution at one particular location or integrated over the entire exit pupil as a function of entrance pupil location rp. In either case we directly obtain OSCE = SCE by insertion of Eq. (7) into Eq. (8). This result agrees best with experiments away from the central fovea (at 1 and 2 deg eccentricity, respectively in Refs. 8 and 18). Possibly, the deviation at the central fovea is related to the illumination of an extended 1 deg retinal patch18 with a resulting increased contribution from larger cone photoreceptors (see Section 6), but it should also be recalled that only limited data are available. Yet another method used to estimate the degree of directionality has been to scan simultaneously (in tandem) both the entrance and the exit pupil. Setting rp = r in Eq. (7) and inserting into Eq. (8) the combined measurement yields OSCE = 3SCE . 共12兲 This is in good agreement with reported values for both the foveal and near-foveal region4 although other studies show an even larger difference (about a factor of 4)14 possibly also due to the illumination of a larger area. As may be noted from Eq. (11), with wp given by Eq. (9), the reported 1 / 2 dependence of the OSCE21 is a direct consequence of diffraction within the present model. Thus, it appears to us that inclusion of scattering from the combined illumination of many photoreceptors is not a prerequisite for reproducing both the wavelength dependence and the narrowing of the OSCE directionality expressed in Eq. (11) as has otherwise been suggested.17,21 Actually, in the case analyzed here the scattering model in which the retinal photoreceptor distribution is compared with that of a rough surface17 cannot be expected to hold valid, as only a single or a few photoreceptors are illuminated at a time by the focused incident beam. In Section 6 we address in more detail the case of a larger illuminated area to elucidate the possible influences that this may have on the analysis. One consequence of the wavelength dependence is that the directionality is easiest seen with a short-wavelength illumination 共short兲 since at a longer wavelength 共long兲 the directional peak will be broader by a factor of approximately 共long / short兲2 in good agreement with experiments.17,21 Interestingly, the variation related to modes7 and spectral properties of the eye34 for the SCE are not seen clearly in experiments on the OSCE, which suggests that the latter is not merely a rescaled reproduction of the SCE. A plausible explanation is that a single reflected mode LP01 dominates the light distribution in the pupil, as also suggested by other authors.14 It should 2322 J. Opt. Soc. Am. A / Vol. 22, No. 11 / November 2005 Vohnsen et al. be borne in mind, however, that the eye and its lens modifies the spectrum of transmitted light in both the ingoing and outgoing pathways. This may distort the above wavelength dependence for the SCE and in a comparable manner for the OSCE. 5. MAXWELLIAN ILLUMINATION CONFIGURATION Our theoretical model has so far been concerned mainly with the problem of illuminating the retina with a tightly focused Gaussian beam as done, e.g., in experiments with the scanning laser ophthalmoscope.4,24 In contrast, most experiments on the SCE and the OSCE use a small entrance at the eye pupil from which the light exposes an extended retinal zone in a Maxwellian illumination system. Thus, at each photoreceptor aperture the incoming light resembles a plane wave. In a first naïve approach Eq. (4) and Eq. (5) may be rewritten to address this problem by assuming that wr Ⰷ wm resulting in 冉 冊 冋 册 冉 冊 冋 册 T共u兲 = 2 T共兲 = 2 wm wr wm 2 wr 2 exp u2 exp − 2 w2r 2 2 wp2 = OSCE . Ttotal共兲 = 兺 冏冕冕 冉 A exp i 2 冊 * dxdy neyex m 冏 2 , 共13兲 . 共14兲 Reading the spread in directionality of the SCE directly from Eq. (14), and assuming the OSCE to remain unchanged by the simultaneous illumination of many photoreceptors [cf. Eq. (11)], one finds SCE = log共e兲 M m=0 , − 2共neyewm兲22 also Section 6). When the illuminated retinal area is large, photoreceptors of different size may contribute to the distribution causing some broadening of the radiation peak.21 Variations in the photoreceptor alignment with respect to the pupil point9 may slightly broaden the summed radiation pattern, but when truly random the distribution will remain rotationally symmetric. When all contributing receptors are well aligned (as in the normal eye) the sum is expected to show the same angular dependence as its individual contributions. Thus, it appears to us that the reason the SCE is approximately twice as broad as the OSCE (in terms of the spread in directionality) should be found rather through a close examination of the photoreceptor-to-light coupling. Generally, the incident (quasi-) plane wave may couple to various modes with the total energy coupled to each photoreceptor given by 共15兲 More generally, if the beam is focused to an area with 1 wr ⬎ wm any value in the range 2 OSCE 艋 SCE 艋 OSCE may be found. This immediately raises the question of why experiments with Maxwellian illumination typically differ from Eq. (15) by the aforementioned factor of 2. This question is best addressed in two parts by considering the wave-guided light returned to the eye pupil separately from light entering the eye. For extended illumination each photoreceptor can be considered to produce a Gaussian beam at the pupil as expressed in Eq. (8) with the distribution for the OSCE obtained as a sum of these (including possible interference effects that in the case of coherent illumination may narrow the distribution17; see 共16兲 where A is the plane-wave amplitude normalized to the area of integration. The sum includes the number of allowed modes 共M + 1兲 as determined by the V number of the inner segment. We have calculated the solutions Ulm of the mode equation in two different inner segments and in the outer segment at two different wavelengths as shown in Table 1 (using the photoreceptor parameters of the previous sections).26 The corresponding coupling efficiency for each mode at different angles of incidence is shown in Table 2. Both tables show numerically obtained values for the exact LP modes without the Gaussian approximation used hitherto for the fundamental mode. In the following, the results are discussed with emphasis on the visible wavelength. It should be noted, however, that for the OSCE a near-IR wavelength might in some ways be preferable since less light gets absorbed in the outer segment (thereby not requiring prebleaching). In turn, the visual sensitivity is very small at the near-IR wavelength making the SCE harder to access, but it simplifies the task of modeling since fewer modes need to be considered. As the angle of incidence is increased, less light couples to the fundamental mode of the inner segment but becomes partially redistributed among higher-order modes. Table 1. Solutions Ulm of the Mode Equation (Ref. 26) for Different Modes LPlm at a Wavelength of 0.543 m (Upper Half) and 0.785 m (Lower Half) a Segment V LP01 LP02 LP11 LP21 di = 2.5 m di = 5.0 m do = 1.0 m 2.7063 5.4126 2.8888 1.7154 2.0216 1.7511 a 2.5914 3.2014 2.6747 4.2547 di = 2.5 m di = 5.0 m do = 1.0 m 1.8720 3.7440 1.9982 1.4824 1.8781 1.5276 Where no value is given the mode is not allowed for the chosen set of parameters. 4.5272 2.9382 Vohnsen et al. Vol. 22, No. 11 / November 2005 / J. Opt. Soc. Am. A 2323 Table 2. Coupling Efficiency of a Plane Wave to Modes LPlm in the Inner and Outer Photoreceptor Segments Calculated at Incident Angles of 0, 5, and 10 deg (in Ascending Order) for a Wavelength of 0.543 m and 0.785 m.a Wavelength 0.543 m Segment LP01 di = 2.5 m 0.851 0.367 0.010 0.691 0.030 0.000 0.836 0.737 0.498 di = 5.0 m do = 1.0 m 0.785 m LP02 LP11 0.168 0.108 0.000 0.000 0.241 0.109 0.000 0.137 0.001 0.000 0.063 0.189 LP21 LP01 LP11 0.000 0.138 0.000 0.924 0.600 0.133 0.775 0.169 0.001 0.913 0.854 0.697 0.000 0.238 0.009 a Obtained from Eq. 共16兲 by integration within a circular area limited by a radius of 0.61d, where d is the respective photoreceptor diameter, as suggested by the diameterto-spacing ratio of 0.82.14,38 The tapered photoreceptor section may redistribute (and reradiate) some of this energy to the modes available in the outer segment. It should be recalled, however, that the SCE is usually studied in unbleached conditions and most light will therefore be detected near the outer segment entrance (assuming a uniform photopigment density) presumably also affected by modes beyond cutoff. Note that even if the outer segment does allow for propagation of the LP11 mode along its entire length (i.e., do ⬎ 1.53), it will not be efficiently excited by backscattered light at small angles (see Table 2). When many modes are allowed in the inner segment, the angle at which each can be efficiently excited can be estimated as lm ⬇ Ulm n id i . 共17兲 This expression has been derived from a ray–optical mode consideration in combination with the Ulm solutions of the waveguide mode equation (its applicability can be appreciated from the individual mode-coupling dependencies shown in Fig. 2).26 For the inner segment with a diameter of 2.5 m it is found from Table 1 [using the values of U01 and U11 in Eq. (17)] that 共11 / 01兲2 ⬇ 2.28 thereby indicating an approximate doubling of the directionality parameter when the mode LP11 is included in the excitation. However, it is also clear that the expression is mainly apt to consider higher-order modes. Thus, to examine the situation in more detail for typical cone sizes, we have calculated the angular dependence of the coupling efficiency, as shown in Fig. 2 for both inner segments. For the smaller segment the sum of excited modes (as well as the fundamental mode) fits accurately a Gaussian distribution. The fitting polynomials may be converted to the form of Eq. (1) using = rp / feye, in which case the corresponding SCE directionality parameters can be found as SCE,01 = 0.102/ mm2 and SCE,sum = 0.048/ mm2. Thus, the simul- Fig. 2. Calculated coupling efficiency T共兲 (thick solid curve) at = 0.543 m for inner segments (a) 2.5 m and (b) 5.0 m including the contributions from individual modes: LP01 (thin solid curve), LP11 (dashed curve), LP21 (dashed–dotted curve), and LP02 (dotted curve). Best-fitted Gaussian distributions versus angle (in radians): (a) T01共兲 = 0.8605 exp关−22 / 0.13182兴, Tsum共兲 = 0.8764 exp关−22 / 0.19182兴, (b) T01共兲 = 0.6980 exp关−22 / 0.07342兴, Tsum共兲 = 0.7831 exp关−22 / 0.14162兴, shown as a series of crosses 共LP01兲 and circles (sum), respectively. 2324 J. Opt. Soc. Am. A / Vol. 22, No. 11 / November 2005 taneous excitation of both modes has lowered the directionality parameter to a similar value as found experimentally. As a consequence of the good fit, Eq. (10) may still be applied provided that the directionality parameter used is that of the sum, viz., SCE = SCE,sum or alternatively that the effective mode width is estimated as wm ⬇ di / U11. When more modes are excited the situation becomes more complex as seen in Fig. 2(b) and the Gaussian fit to the sum becomes less accurate. Nonetheless, it reveals directionality parameters for the large inner segment of SCE,01 = 0.327/ mm2 and SCE,sum = 0.088/ mm2, respectively. Thus, the fit for the sum is in good agreement with the experimentally found value at the parafoveal retina.35,36 For light exiting the eye, it may be noted that the directionality parameter is identical with that of the fundamental mode for the SCE, viz., OSCE = SCE,01 provided that the back-reflected light distribution is dominated by the LP01 mode as discussed above for the smaller segment and as suggested by other authors.14 Under this condition, the width of photoreceptors contributing to the OSCE may still be estimated as in Section 4, i.e., with the inner segment diameter estimated by di ⬇ V0wm and with the fundamental mode width obtained by means of Eq. (11). It may be noted that the observed increase of the ratio OSCE / SCE ⬃ SCE,01 / SCE,sum with foveal distance is in qualitative agreement with observations (see Fig. 7 in Ref. 12). The nontrivial behavior observed with a scanning system for OSCE as a function of foveal distance, increasing to an eccentricity of ⬃2° beyond which it smoothly decays,4 may also be explained in the context of the present model. At and near the fovea the situation resembles that of the Maxwellian illumination configuration with simultaneous excitation of more modes as described above, but at larger eccentricities the focused beam produces a spot at the retina comparable with (Section 4) or smaller than the typical cone photoreceptor resulting in a decrease of the directionality parameter [i.e., Eq. (5) with wr ⬍ wm]. The angular dependence of the coupling efficiency may be examined in a similar manner at other wavelengths, and specific results obtained with near-IR are shown in Fig. 3. Here it should be noted that the inner segment with a 2.5 m diameter allows propagation only of the fundamental mode. Thus, in this case Gaussian fitting leads to SCE,01 = SCE,sum = 0.053/ mm2, whereas for the inner segment with a 5.0 m diameter it can be found that SCE,01 = 0.176/ mm2 and SCE,sum = 0.087/ mm2. With regard to the wavelength dependence of the directionality parameter, it may be observed that the ratio OSCE 共 0.785 m 兲 / OSCE 共 0.543 m 兲 ⬃SCE,01共0.785 m兲 / SCE,01共0.543 m兲 equals ⬃0.52 for the smaller and ⬃0.54 for the larger inner segment, and is therefore close to the expected value of 共0.543/ 0.785兲2 ⬇ 0.48. The small deviation from a 1 / 2 dependence is due to the quality of fitting as well as a minor wavelength dependence of the exact mode width. The same wavelength relationship is not revealed from the fitting of the SCE 共SCE ⬃ SCE,sum兲 because of the changed number of allowed modes. Here it appears only within a given wavelength range when the number of allowed modes is constant.7 This indicates that the OSCE might represent the better way of accessing photoreceptor data when the actual number of allowed Vohnsen et al. Fig. 3. Calculated coupling efficiency T共兲 (thick solid curve) at = 0.785 m for inner segments (a) 2.5 m and (b) 5.0 m including the contributions from individual modes: LP01 (thin solid curve) and LP11 (dashed curve). Best-fitted Gaussian distributions versus angle (in radians): (a) T01共兲 = Tsum共兲 = 0.9310 ⫻exp关−22 / 0.18322兴, (b) T01共兲 = 0.7833 exp关−22 / 0.10012兴, Tsum共兲 = 0.8062 exp关−22 / 0.14252兴, shown as a series of crosses 共LP01兲 and circles (sum), respectively. modes is not known a priori (which is normally the case). A central concern with regard to the modeling of the SCE and the OSCE is how sensitive the estimations are to specific model details and to uncertainties in the implemented parameters. On the one hand, a strong sensitivity holds promise of accurate measurements of physical parameters noninvasively, but on the other it may complicate a direct validation of the model. One issue is the actual step-index fiber model chosen on the basis of the fact that modes (including those of higher order) observed near the outer segment termination of in vitro human photoreceptors resemble strongly the modes used in the present model.6 In turn, experiments on certain fish photoreceptors have rather suggested a graded-index model.39 Actually, in the present context a similar feature would not be of major concern since the Gaussian mode approximation is exact for the fundamental mode in a parabolic index distribution (and highly similar for other index distributions).26 Another issue is the reported wavelength dependence of the SCE for a single photoreceptor where modeling seems to produce too abrupt changes (apart from the overall 1 / 2 factor) as the number of allowed modes change.7 Vohnsen et al. Presumably these features are not prominent experimentally due to an averaging and smoothing effect when illuminating an extended retinal patch (containing slightly different photoreceptors). Actually, with simultaneous illumination of several photoreceptors, as in standard experiments on the SCE, the larger ones will collect a larger fraction of the incident light and may therefore have stronger influence on the visual response. A proper weighting of contribution photoreceptors in Maxwellian illumination systems may therefore lead to even better numerical agreement between simulated and histological data. Moreover, because of its small length the inner segment cannot be expected to have an abrupt mode cutoff in transmission. Thus, even beyond cutoff some light may still reach the outer segment and contribute to the sensed level of brightness. With respect to the influence of parameter uncertainties (refractive indices, waveguide diameters) on the predictions of the model, these can perhaps best be appreciated through the V number of the inner segment. Since any such deviation would produce a corresponding change in V, the spectral regions where a given number of modes would be allowed can shift and thereby influence the SCE directionality parameter (standard experiments would average such details among a given number of illuminated photoreceptors).7 Thus, only if some of these variables are accurately known by other means does the SCE appear to be suitable for probing for additional information. In turn, the present model suggests that the OSCE may be used to directly access the photoreceptor diameter through the relationship di ⬀ 共OSCE兲0.5. Such knowledge may subsequently be used as input to further studies with the SCE. It should be emphasized, that the relation between the OSCE directionality parameter and photoreceptor diameter found in the present study differs from that of the aforementioned retinal scattering model in which it was rather related to the average photoreceptor spacing.17,18 However, since both the inner segment diameter and average cone spacing increase with foveal distance, this may complicate a direct experimental verification in favor of either model. 6. COHERENT VERSUS INCOHERENT ILLUMINATION AND THE IMPORTANCE OF PHASE For simplicity of discussion no direct reference to the state of coherence of the illumination has yet been made (although monochromatic coherent light has implicitly been assumed). Historically, the first experiments on the SCE were performed with incoherent sources whereas later experiments on both the SCE and the OSCE have often made use of highly coherent laser light. It is thus fair to ask whether this may have any significant influence on either effect and if this can be estimated within the present model. In the following the possible implications of coherence are discussed first for the SCE and subsequently for the OSCE. For standard experiments on the SCE no such difference ought to be observable since the angle of incidence on the retina is the same in both cases and only the trans- Vol. 22, No. 11 / November 2005 / J. Opt. Soc. Am. A 2325 mitted power [see Eq. (16)] matters for the visual response at any given moment. This is also in agreement with experimental findings.40 However, if simultaneous illumination from different parts of the pupil is permitted this need no longer be the case since only a coherent field may interfere in the retinal plane. In this context the possible additivity of the SCE (i.e., whether different parts of the illumination will combine to reinforce the SCE or whether they may interact to either partially or completely nullify the effect) is of central concern. In one such study, indeed a reduced contribution (although slightly less than expected) from light near the rim of a large pupil was found with incoherent light as predicted by the SCE with additivity.41 Such knowledge has been applied to the design of pupil filters that neutralize the SCE.2 Another study whose object was a direct comparison with standard experiments on the SCE made use of a sectioned annular pupil aperture (with mean radius rp) so that all light within the eye was largely confined within a cone shell having the retina at its apex.42 With the field added coherently and in phase from the entire annular aperture, the resulting wavefront at the retina should show no tilt and therefore no SCE. The field at the retina can be expressed via a Fourier transform of the pupil field, i.e., r共x , y兲 ⬀ F兵Pa共x , y兲exp关ir共x , y兲兴其 = F兵Pa共x , y兲其 丢 F兵exp关ir共x , y兲兴其. Here Pa denotes the magnitude of the field transmitted by the annular aperture and r the corresponding phase distribution that may be taken to include also ocular aberrations. The symbol 丢 refers to a convolution. Clearly, if r is not constant on the entire aperture any phase distribution on the retina may result (different from that of F兵Pa共x , y兲其 alone). Thus, both ocular aberrations and phase variations in the incident light may have a detrimental effect and hamper the interpretation of the experimental outcome. In the referenced study an incoherent source was used in conjunction with a diffuser (introducing phase variations) and it was concluded that the SCE was additive. However, in our opinion the experimental evidences referred to do not suffice to reject the present hypothesis of amplitude field addition on which Eq. (3) is based. Rather, a conclusive test should be performed with a highly coherent source and a well-controlled wavefront at the entire pupil plane. With an incoherent source, interference in the retinal plane would cancel as a result of random phase fluctuations in the illumination and the expected result would be that of the standard SCE. To examine the possible influence on the OSCE of illuminating a large retinal patch (containing numerous photoreceptors) with either coherent or incoherent light, the variation in photoreceptor length and consequently the phase of the reflected wave-guided light should be considered.17 Actually, if no phase differences are produced for coherent light one should expect only a tiny bright spot near the center of the pupil (toward which all photoreceptors are oriented9), since in this case the light reradiated from the retina would add coherently to resemble a convergent spherical wavefront focused at the pupil. Since this is not what is seen experimentally, the phase of the reflected light must play an important role. To describe its effect, the pupil field may be written as a sum of Gaussian beams originating at each photoreceptor 2326 J. Opt. Soc. Am. A / Vol. 22, No. 11 / November 2005 Vohnsen et al. j with different phase shifts j. The intensity produced at the pupil can thus be written as 冋 Idir共x,y兲 = I0TR exp − 冏兺 2共x2 + y2兲 wp2 册 N ⫻ j=1 exp关i共kx,jx + ky,jy + j兲兴 冏 2 , 共18兲 where kxj and kyj represent the wave-vector components in the pupil plane for light originating at each contributing photoreceptor. With only one photoreceptor the expression reduces to that of Eq. (8). For simplicity it has been assumed that all N photoreceptors radiate equally brightly and have the same mode width (diameter). If this were not the case, the Gaussian weighting factor would be slightly different for each and should therefore act within the summation sign to produce a different weighting of each plane-wave component. Clearly, this could slightly modify the observed directionality parameter as already mentioned. With random phase shifts j distributed uniformly (when wrapped) on the interval 关0 ; 2关, the summation term of Eq. (18) approaches the well-known random walk at any pupil point 共x , y兲. Thus, for coherent light the intensity at each pupil point may take on any value from zero to the maximum allowed (due to constructive interference), giving rise to a speckle pattern. It should be noted, however, that the speckle pattern contained in the summation is in itself not spatially confined [see Fig. 4(b)]. The only spatial confinement is due to the Gaussian weighting that is the same as in Eq. (8). Other authors have argued for a normal distribution of the phase terms in order to achieve a spatial confinement,17 but in the present model it is simply due to diffraction. For incoherent light no interference is observable and the summation in Eq. (18) can be replaced by unity. Experimental results have shown some difference between the coherent10–12,14,17,18 and the incoherent case20,21 which has been attributed to the difference in size of the illuminated areas. However, the available data are still very few, and have been obtained under different experimental conditions, making further experiments highly desirable. In Fig. 4 we have simulated the development of an illuminated extended retinal patch and the gradual approximation of averaged pupil intensity distributions (each having a random phase at each photoreceptor) to the distribution for incoherent light. An alternative to averaging numerous similar pupil images is the calculation of an averaged radial distribution within a single image, although this would require an accurate determination of the pupil center. The retinal curvature with all receptors pointed toward a single pupil point,9 and the fact that wave-guided light contributes to the intensity distribution at the pupil means that a coherent retinal scattering model for a corrugated surface17 (where each local facet points in an arbitrary direction) does not seem entirely satisfactory in our view. In turn, the present description gives both directionality parameters and wavelength dependence within the scope of a single model. In this context, it should be stressed that diffraction is a necessity to explain how light is radiated from tiny structures. This may also explain why scattering models give the same wavelength dependence, as they too are based on the diffraction of propagating wave fields. 7. CONCLUSIONS Fig. 4. Simulated OSCE at an illumination wavelength of 0.543 m of (a) 1° uniformly illuminated retinal patch with 1221 randomly located contributing cones (i.e., a density of 20,000 cones/ mm2) and fundamental mode width wm = 1.35 m (for simplicity partial cone overlap has not been prohibited in the model). The coherent plane-wave contribution [i.e., the unweighted sum of Eq. (18)] is shown in (b) at the pupil plane 关6 ⫻ 6 mm2兴. Corresponding intensity distributions for a 6 mm pupil are shown for coherent light with (c) 1, (d), 10, (e) 100 averaged frames and compared with the case of (f) incoherent light. In this paper we have extended the common waveguide theory of photoreceptors7,14 to formulate a theory of both the SCE and the OSCE. This has been done analytically within a Gaussian mode approximation and numerically for the LP modes. Our description differs from others by including diffraction for the propagation between eye pupil and photoreceptor aperture for both incoming and back-reflected light. Despite the small size of photoreceptors, the diffraction in relation to waveguide coupling has to our knowledge not been considered previously. The present description is valid for describing illumination of a single photoreceptor as well as an extended retinal patch containing numerous photoreceptors. The Gaussian approximation has simplified the description and allowed us to derive valuable analytical expressions for the direc- Vohnsen et al. tionality parameters used in the description of both the SCE and the OSCE. We showed explicitly that the commonly observed difference of a factor of 2 for the directionality parameter, as observed with the two techniques in Maxwellian illumination systems, can be explained by the difference in coupling of the incident and back-reflected light. Whereas the incident light may couple to various modes, the fundamental mode governs the back-reflected light as suggested by the OSCE. This observation is in agreement with previous predictions by others.14 The present model also reproduces the overall wavelength dependence of the directionality parameter,19–21 here identified as a diffraction feature of the waveguided light for the OSCE, and the larger value for the directionality parameter reported when scanning simultaneously both entrance and exit pupil.4,14 More experiments, at different wavelengths and with both coherent and incoherent light, are still needed in order to address directly the SCE and the OSCE and thereby evaluate the validity of the present model. Eventually, such studies may permit in vivo deduction of photoreceptor parameters such as mode width and inner segment diameter at different retinal locations. Finally, experiments may also serve to elucidate the influence of other parameters, such as wavefront aberrations and rod contributions, as well as the influence of a background illumination [C ⫽ 0 in Eq. (1)], which can affect the observed directionality parameters as well as the quality of cone-mosaic imaging with high-resolution ophthalmoscopy.24,43 ACKNOWLEDGMENTS The authors gratefully acknowledge financial support from the Ministerio de Educación y Ciencia, Spain (grant BFM2001-0391 and FIS2004-02153) and Ramón y Cajal research contract RYC2002-006337. The authors are also grateful for a number of valuable suggestions received from two reviewers and JOSA A Editor Stephen A. Burns. Corresponding author Brian Vohnsen’s e-mail address is vohnsen@um.es. Vol. 22, No. 11 / November 2005 / J. Opt. Soc. Am. A 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. REFERENCES AND NOTES 1. 2. 3. 4. 5. 6. 7. 8. 9. W. S. Stiles and B. H. Crawford, “The luminous efficiency of rays entering the eye pupil at different points,” Proc. R. Soc. London 112, 428–450 (1933). D. A. Atchison, D. H. Scott, N. C. Strang, and P. Artal, “Influence of Stiles–Crawford apodization on visual acuity,” J. Opt. Soc. Am. A 19, 1073–1083 (2002). G. Toraldo di Francia and L. Ronchi, “Directional scattering of light by the human retina,” J. Opt. Soc. Am. 42, 782–783 (1952). P. J. Delint, T. T. J. M. Berendschot, and D. van Norren, “Local photoreceptor alignment measured with a scanning laser ophthalmoscope,” Vision Res. 37, 243–248 (1996). B. O’Brien, “A theory of the Stiles and Crawford effect,” J. Opt. Soc. Am. 36, 506–509 (1946). J. M. Enoch, “Optical properties of the retinal receptors,” J. Opt. Soc. Am. 53, 71–85 (1963). A. W. Snyder and C. Pask, “The Stiles–Crawford effect— explanation and consequences,” Vision Res. 13, 1115–1137 (1973). A. Roorda and D. R. Williams, “Optical fiber properties of individual human cones,” J. Vision 2, 404–412 (2002). A. M. Laties and J. M. Enoch, “An analysis of retinal receptor orientation,” Invest. Ophthalmol. Visual Sci. 10, 69–77 (1971). S. A. Burns, S. Wu, F. Delori, and A. E. Elsner, “Direct measurement of human-cone-photoreceptor alignment,” J. Opt. Soc. Am. A 12, 2329–2338 (1995). S. A. Burns, S. Wu, J. C. He, and A. E. Elsner, “Variations in photoreceptor directionality across the central retina,” J. Opt. Soc. Am. A 14, 2033–2040 (1997). J. C. He, S. Marcos, and S. A. Burns, “Comparison of cone directionality determined by psychophysical and reflectometric techniques,” J. Opt. Soc. Am. A 16, 2363–2369 (1999). R. A. Applegate and V. Lakshminarayanan, “Parametric representation of Stiles–Crawford functions: normal variation of peak location and directionality,” J. Opt. Soc. Am. A 10, 1611–1623 (1993). J.-M. Gorrand and F. C. Delori, “A model for assessment of cone directionality,” J. Mod. Opt. 44, 473–491 (1997). A. Safir and L. Hyams, “Distribution of cone orientations as an explanation of the Stiles–Crawford effect,” J. Opt. Soc. Am. 59, 757–765 (1969). G. J. van Blokland, “Directionality and alignment of the foveal receptors, assessed with light scattered from the human fundus in vivo,” Vision Res. 26, 495–500 (1986). S. Marcos, S. A. Burns, and J. C. He, “Model for cone directionality reflectometric measurements based on scattering,” J. Opt. Soc. Am. A 15, 2012–2022 (1998). S. Marcos and S. A. Burns, “Cone spacing and waveguide properties from cone directionality measurements,” J. Opt. Soc. Am. A 16, 995–1004 (1999). W. S. Stiles, “The luminous efficiency of monochromatic rays entering the eye pupil at different points and a new colour effect,” Proc. R. Soc. London, Ser. B 123, 90–118 (1937). N. P. A. Zagers, J. van de Kraats, T. T. J. M. Berendschot, and D. van Norren, “Simultaneous measurement of foveal spectral reflectance and cone-photoreceptor directionality,” Appl. Opt. 41, 4686–4696 (2002). N. P. A. Zagers, T. T. J. M. Berendschot, and D. van Norren, “Wavelength dependence of reflectometric cone photoreceptor directionality,” J. Opt. Soc. Am. A 20, 18–23 (2003). A. W. Snyder, “Excitation of waveguide modes in retinal receptors,” J. Opt. Soc. Am. 56, 705–706 (1966). M. J. Piket-May, A. Taflove, and J. B. Troy, “Electrodynamics of visible-light interactions with the vertebrate retinal rod,” Opt. Lett. 18, 568–570 (1993). B. Vohnsen, I. Iglesias, and P. Artal, “Directional imaging of the retinal cone mosaic,” Opt. Lett. 29, 968–970 (2004). In the case that the incoming field at the retina r and the mode field m have not been normalized before performing the integration in Eq. (3), the normalization can be performed on the entire expression by replacing the equation with Pm Pr 26. 2327 = 冕冕 冏冕冕 * r m dxdy 兩r兩 dxdy 2 冕冕 冏 2 . 兩m兩 dxdy 2 Just as before this expression gives the percentage of incident power coupled to the guided mode m. The total amount of guided power is found by adding up the contributions Pm from each allowed mode. Clearly, any single photoreceptor will carry only a tiny fraction of the total incident light power Pr unless the incident field is strongly confined at the photoreceptor. In turn, the guided modes are strongly localized at each waveguide and their area of normalization therefore need only to be slightly larger than the waveguide diameter. Note that the mode nomenclature of a weakly guiding stepindex fiber has been chosen. Here the fundamental mode 2328 J. Opt. Soc. Am. A / Vol. 22, No. 11 / November 2005 LP01 is rotationally symmetric and can be represented by zeroth order Bessel functions J0共2U01r / di兲 in the core and 2 r / di兲 in the cladding (U01 is the fundamental K0共2冑V2 − U01 solution of the mode equation). Generally, the modes are found by solving the equation Ulm 27. 28. 29. 30. 31. 32. Jl+1共Ulm兲 Jl共Ulm兲 2 = 冑V2 − Ulm 2 Kl+1共冑V2 − Ulm 兲 2 Kl共冑V2 − Ulm 兲 numerically for the unknown Ulm, where Jl is the Bessel function of the first kind and Kl is the modified Bessel function of the second kind both of order l (see also pp. 135–137 in Ref. 27). The fundamental mode profile 共l = 0 , m = 1兲 strongly resembles a Gaussian distribution function exp共 −r2 / wm2兲 and the same holds true for the fundamental mode of fibers with other index distributions (see Fig. 8.12 in Ref. 27). A. K. Ghatak and K. Thyagarajan, Introduction to Fiber Optics (Cambridge U. Press, 1998), pp. 149–156. B. Chen and W. Makous, “Light capture by human cones,” J. Physiol. (London) 414, 89–109 (1989). J. van de Kraats, T. T. J. M. Berendschot, and D. Van Norren, “The pathways of light measured in fundus reflectometry,” Vision Res. 36, 2229–2247 (1996). G. J. van Blokland and D. van Norren, “Intensity and polarization of light scattered at small angles from the human fovea,” Vision Res. 26, 485–494 (1986). J. A. Van Loo, Jr. and J. M. Enoch, “The scotopic Stiles– Crawford effect,” Vision Res. 15, 1005–1009 (1975). O. Packer, D. G. Bensinger, and D. R. Williams, “In vitro angular tuning of single primate rods and cones and the Stiles–Crawford effect [abstract],” Invest. Ophthalmol. Visual Sci. 35, 1572 (1994). Vohnsen et al. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. C. Pask and A. Stacey, “Optical properties of retinal photoreceptors and the Campbell effect,” Vision Res. 38, 953–961 (1998). T. T. J. M. Berendschot, J. van de Kraats, and D. Van Norren, “Wavelength dependence of the Stiles–Crawford effect explained by perception of backscattered light from the choroid,” J. Opt. Soc. Am. A 18, 1445–1451 (2001). G. Westheimer, “Dependence of the magnitude of the Stiles–Crawford effect on retinal location,” J. Physiol. (London) 192, 309–315 (1967). J. M. Enoch and G. M. Hope, “Directional sensitivity of the foveal and parafoveal retina,” Invest. Ophthalmol. Visual Sci. 12, 497–503 (1973). G. Toraldo di Francia, “Retina cones as dielectric antennas,” J. Opt. Soc. Am. 39, 324 (1949). C. A. Curcio, K. R. Sloan, R. E. Kalina, and A. E. Hendrickson, “Human photoreceptor topography,” J. Comp. Neurol. 292, 497–523 (1990). M. P. Rowe, N. Engheta, S. S. Easter, Jr., and E. N. Pugh, Jr., “Graded-index model of a fish double cone exhibits differential polarization sensitivity,” J. Opt. Soc. Am. A 11, 55–70 (1994). L. J. Bour and J. C. M. Verhoosel, “Directional sensitivity of photoreceptors for different degrees of coherence and directions of polarization of the incident light,” Vision Res. 19, 717–719 (1979). J. M. Enoch, “Summated response of the retina to light entering different parts of the pupil,” J. Opt. Soc. Am. 48, 392–405 (1958). B. Drum, “Additivity of the Stiles–Crawford effect for a Fraunhofer image,” Vision Res. 15, 291–298 (1975). B. Vohnsen, I. Iglesias, and P. Artal, “Directional light scanning laser ophthalmoscope,” J. Opt. Soc. Am. A 22(12) (2005).