Belt of Stability e0 Sn In + → Belt of Stability I Xe → + e
advertisement
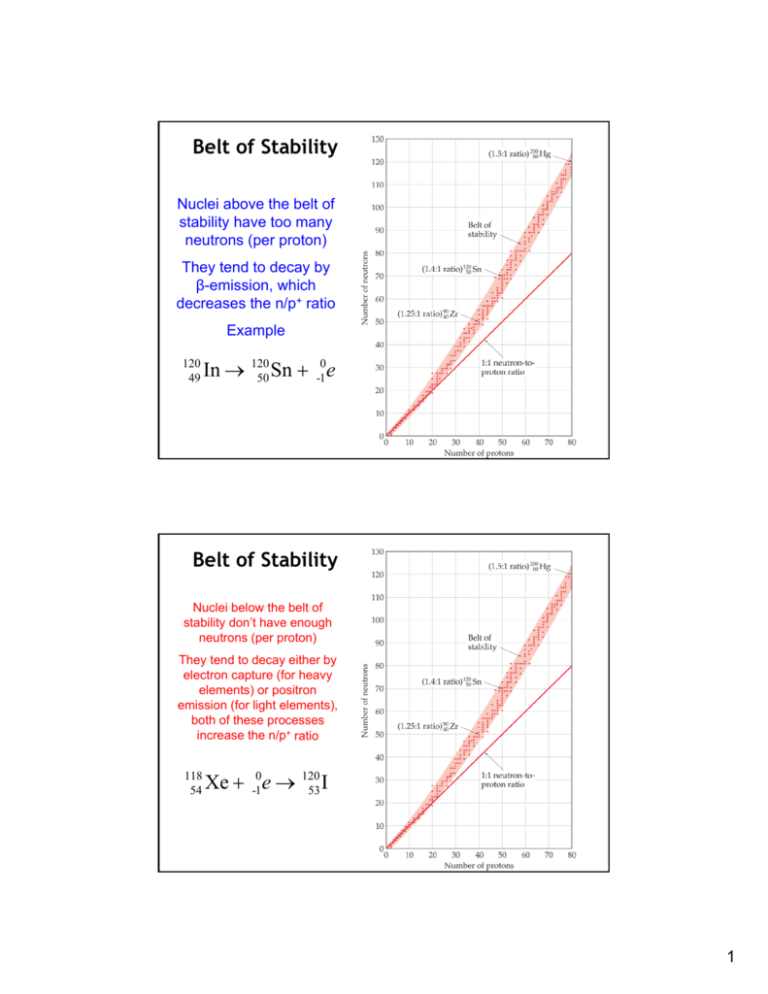
Belt of Stability Nuclei above the belt of stability have too many neutrons (per proton) They tend to decay by β-emission, which decreases the n/p+ ratio Example 120 49 In → 120 50 Sn + -10e Belt of Stability Nuclei below the belt of stability don’t have enough neutrons (per proton) They tend to decay either by electron capture (for heavy elements) or positron emission (for light elements), both of these processes increase the n/p+ ratio 118 54 Xe + -10e → 120 53 I 1 Belt of Stability Nuclei with atomic numbers greater than 83 are all unstable. They have too many protons and neutrons. They tend to decay by α-emission 239 94 Pu → 237 92 U + 42 He Radioactive Series Large radioactive nuclei cannot stabilize by undergoing only one nuclear transformation. They undergo a series of decays until they form a stable nuclide (often a nuclide of lead). This example shows a series of α- and β-decays that start with 238U and end with 206Pb 2 Other Considerations Nuclei with 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons tend to be more stable than nuclides with a different number of nucleons. Radioactive Decay – Half Life 3 Binding Energy per Nucleon Elements with intermediate masses have the highest nuclear binding energies Chain Reaction 4 Nuclear Weapons 5 Nuclear Reactor Nuclear Power Plant 6 Nuclear Fusion 7