•
•
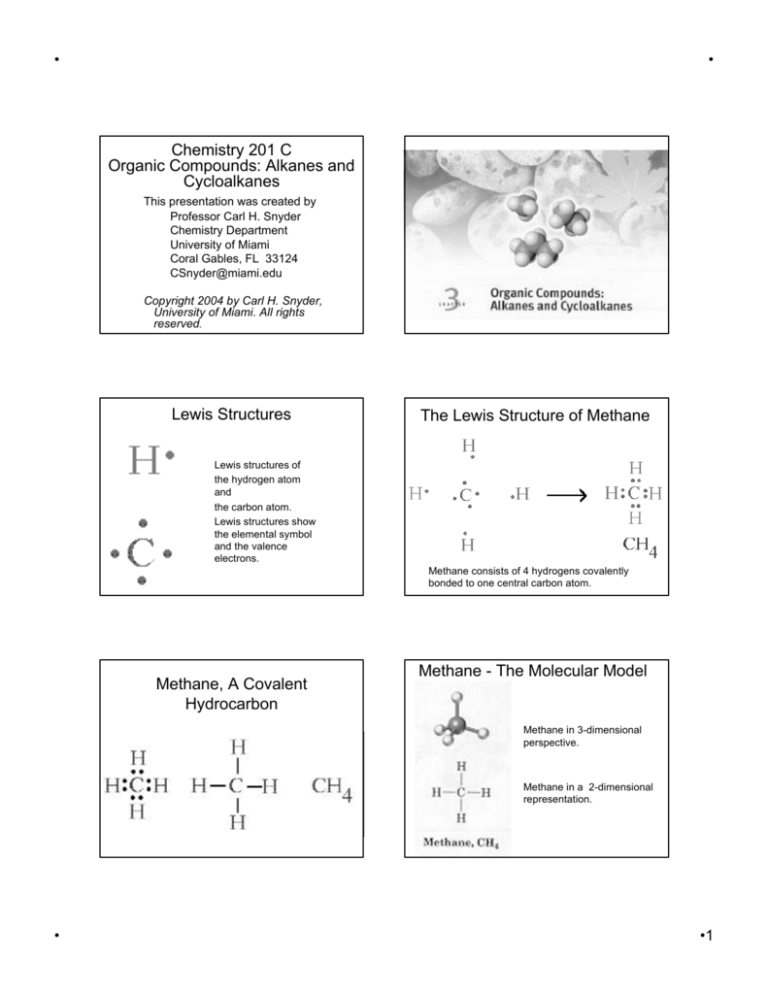
Chemistry 201 C
Organic Compounds: Alkanes and
Cycloalkanes
This presentation was created by
Professor Carl H. Snyder
Chemistry Department
University of Miami
Coral Gables, FL 33124
CSnyder@miami.edu
Copyright 2004 by Carl H. Snyder,
University of Miami. All rights
reserved.
Lewis Structures
The Lewis Structure of Methane
Lewis structures of
the hydrogen atom
and
the carbon atom.
Lewis structures show
the elemental symbol
and the valence
electrons.
Methane consists of 4 hydrogens covalently
bonded to one central carbon atom.
Methane, A Covalent
Hydrocarbon
Methane - The Molecular Model
Methane in 3-dimensional
perspective.
Methane in a 2-dimensional
representation.
•
•1
•
•
The Methyl Radical
methyl radical
A Radical
In chemistry, a radical is any species
bearing an unpaired electron.
Loss of a hydrogen atom from methane
produces the methyl radical, also known as
the methyl free radical.
Note that loss of any one of methane’s four
hydrogens produces the same methyl radical.
Ethane From Two Methyl
Radicals
Ethane - Expanded Structure,
Condensed Structure, And
Molecular Formula
Expanded structure
The combination of
two methyl radicals
forms ethane.
Ethane can form in
many other ways as
well.
Condensed structure
Molecular formula
Ethane - The Molecular Model
The Ethyl Radical
Ethane in 3-dimensional
perspective.
the ethyl radical
Ethane in a 2-dimensional
representation.
•
Loss of a hydrogen atom from ethane produces
the ethyl radical, also known as the ethyl free
radical.
Notice that loss of any one of ethane’s six
hydrogens produces the same ethyl radical.
•2
•
•
Propane From An Ethyl
Radical And A Methyl Radical
Propane - Expanded Structure,
Condensed Structure, And
Molecular Formula
Expanded structure
The combination of an ethyl radical and a
methyl radical produces propane.
Propane can form in many other ways as well.
Condensed structure
Molecular formula
Propane - The Molecular Model
Propane in 3-dimensional
perspective.
Propane, a 2-dimensional
representation.
The First Members of The
Alkane Series
All compounds whose molecular formulas
fit the general formula CnH(2n+2) are
alkanes
•
Alkanes and Alkyl Groups
Methane, ethane, and propane are
members of a group of hydrocarbons
known as the alkanes.
Removing a hydrogen atom from an
alkane produces an alkyl group.
CH3- is the methyl group, as in methyl
chloride, CH3-Cl ; CH3-CH2- is the ethyl
group, as in ethyl chloride, CH3-CH2-Cl
Alkyl groups are indicated generically by
the symbol R-, as in R-Cl
Some Terms to Remember
Hydrocarbon - A compound composed
exclusively of hydrogen and carbon.
Saturated hydrocarbon - A compound of
general formula CnH(2n+2) containing the
maximum possible number of hydrogens
per carbon.
Alkane - The family of compounds with the
general formula CnH(2n+2); the saturated
hydrocarbons.
Aliphatic hydrocarbons - An older term for
the alkane family, derived from a Greek
word for "fat" or "fatty substance"
•3
•
•
Classes of Carbons
Primary (1o) and Secondary (2o)
Carbons A primary (1o)
carbon is
bonded to
exactly one
other carbon.
A secondary
(2o) carbon is
bonded to
exactly two
other carbons
1o
And
2o
Hydrogens In Propane
1 - 1o carbon
1 - 1o carbon
Classes of Hydrogens
Hydrogens always take the same class as
the carbons to which they are bonded.
All hydrogens on a primary carbon are
primary hydrogens.
All hydrogens on a secondary carbon are
secondary hydrogens.
1o And 2o Hydrogens In Propane
1 - 1o carbon
3 - 1o hydrogens
Propane contains two 1o carbons and . . .
1 - 1o carbon
3 - 1o hydrogens
Propane contains two 1o carbons and six
1o hydrogens, and . . .
1o And 2o Hydrogens In Propane
1o And 2o Hydrogens In Propane
1 - 1o carbon
1 - 1o carbon
1 - 2o carbon
1 - 1o carbon
1 - 1o carbon
1 - 2o carbon
2 - 2o hydrogens
3 - 1o hydrogens
3 - 1o hydrogens
Propane contains two
1o hydrogens, and
one 2o carbon and . . .
•
1o
carbons and six
3 - 1o hydrogens
3 - 1o hydrogens
Propane contains two 1o carbons and six
1o hydrogens, and
one 2o carbon and two 2 o hydrogens.
•4
•
•
Two Different Propyl Radicals
primary propyl radical
Two Different Propyl Radicals
primary propyl radical
secondary propyl radical
Removing a 1o H produces a 1o propyl radical.
The Propyl Radical and the
Isopropyl Radical
Removing a 1o H produces a 1o propyl radical.
Removing a 2o H produces a 2o propyl radical.
Two Different Butanes
The addition of a methyl group to the 1o carbon
of a propyl radical produces a straight-chain
C4H10.
Two Different Butanes
Isomers
Different compounds that share the same
molecular formula are known as isomers.
The straight-chain structure and the branchedchain structure represent the two isomers of
butane, C4H10.
The addition of a methyl group to the 1o carbon of a
propyl radical produces a straight-chain C4H10.
The addition of a methyl group to the 2o carbon of
an isopropyl radical produces a branched-chain
C4H10.
•
•5
•
•
The Two Isomers of Butane
The Tertiary Carbon And The
Tertiary Hydrogen Of Isobutane
A tertiary (3o) carbon
is bonded to exactly
three other carbons.
The hydrogen on the
tertiary carbon is a
tertiary hydrogen.
Although each structure represents one of the
isomers of butane, C4H10, the straight-chain
structure is known as butane, and
the branched-chain structure is known as
isobutane.
The Tertiary Carbon And The
Tertiary Hydrogen Of Isobutane
The Tertiary Carbon And The
Tertiary Hydrogen Of Isobutane
A tertiary (3o) carbon
is bonded to exactly
three other carbons.
The hydrogen on the
tertiary carbon is a
tertiary hydrogen.
A tertiary (3o) carbon
is bonded to exactly
three other carbons.
The hydrogen on the
tertiary carbon is a
tertiary hydrogen.
3o carbon
They’re All Butane, C4H10
Each of these structures represents butane, the
straight-chain, unbranched isomer.
As long as you can pass from one end of the
chain to the other end without encountering a
branch, it’s butane.
3º H
The Three Isomers of Pentane,
C5H12
pentane
pentane
Pentane
•
3o carbon
isopentane
isopentane
Isopentane
neopentane
Neopentane
•6
•
•
The Quaternary Carbon of
Neopentane
A quaternary (4o)
carbon is bonded to
exactly four other
carbons.
Because all of its
valences are
consumed in bonding
to other carbons, a
quaternary carbon
cannot be bonded to a
hydrogen.
Quaternary hydrogens
do not exist.
quaternary
carbon
quaternary
carbon
The First 10 Alkanes
We are concerned with the names of the first
10 alkanes.
Those with five or more carbons begin with
terms derived from Latin or Greek words
for the numbers one through ten.
They end with -ane to specify that we are
dealing with alkanes.
Alkyl Groups:
The Two C3 Alkyl Groups
CH3-CH2-CH2-Cl is propyl chloride
CH3-CH-CH3 is isopropyl chloride
|
Cl
•
The Four C4 Alkyl Groups
CH3-CH2-CH2-CH2-Cl is butyl chloride
CH3-CH2-CH-CH3 is sec-butyl chloride
|
Cl
•7
•
•
The Four C4 Alkyl Groups
CH3-CH-CH2-Cl is isobutyl chloride
|
CH3
Alkane Isomers
The enormous number of isomers of alkanes and alkyl
groups overwhelms systems that use prefixes such as
iso- and neo-.
The IUPAC system can accommodate large numbers of
isomers.
IUPAC
•
The Four C4 Alkyl Groups
CH3
|
CH3-C-Cl is tert-butyl chloride
|
CH3
The IUPAC (International Union of
Pure and Applied Chemistry)
System
IUPAC
•8
•
•
IUPAC
IUPAC
IUPAC
IUPAC
IUPAC
IUPAC
•
•9
•
•
•
IUPAC
IUPAC Example #1
IUPAC Example #1
IUPAC Example #1
IUPAC Example #1
IUPAC Example #2
•10
•
•
•
IUPAC Example #2
IUPAC Example #2
IUPAC Example #2
IUPAC Example #3
IUPAC Example #3
IUPAC Example #3
•11
•
•
IUPAC Example #3
IUPAC Example #4
IUPAC Example #4
IUPAC Example #4
IUPAC Example #4
IUPAC Example #5
•
•12
•
•
IUPAC Example #5
IUPAC Example #5
IUPAC Example #5
Cycloalkanes
CH2
CH2 CH2
CH2
CH2
CH2
CH2
CH2
CH2
Naming Cycloalkanes
•
Naming Cycloalkanes
•13
•
•
•
Naming Cycloalkanes
Naming Cycloalkanes
Cycloalkanes - Example #1
Cycloalkanes - Example #1
Cycloalkanes - Example #1
Cycloalkanes - Example #1
•14
•
•
Cycloalkanes - Example #2
Cycloalkanes - Example #2
Cycloalkanes - Example #3
•
Cycloalkanes - Example #2
Cycloalkanes - Example #3
Cycloalkanes - Example #3
•15
•
•
Kinds of Isomerism
Cycloalkanes - Example #3
The Rigid Ring of
Cyclopropane
Constitutional isomers show different sequences in
the connections of their atoms - butane and isobutane
are constitutional isomers.
Stereoisomers show the same sequences of
connections, but differ in the 3-dimensional, spacial
orientation of their atoms.
cis-1,2-Dimethylcyclopropane
The rigid ring of cyclopropane fixes substituents
on each side of the ring, allowing the kind of
stereoisomerism known as geometric or
cis/trans isomerism.
The two CH3 groups of cis-1,2dimethylcyclopropane lie on the same side of
the ring.
Moving one of the CH3 groups to the other side
of the ring would require breaking covalent
bonds, which is a high-energy process.
trans-1,2Dimethylcyclopropane
The two CH3 groups of trans-1,2dimethylcyclopropane lie on opposite sides of
the ring.
Moving one of the CH3 groups to the other side
of the ring would require breaking covalent
bonds, which is a high-energy process.
•
Configurational Isomers
If interconverting isomers (as in the
case of stereoisomers) requires the
breaking and reforming of covalent
bonds, the isomers are known as
configurational isomers.
•16
•
•
End
Organic Compounds: Alkanes and
Cycloalkanes
•
•17