How Much Water Fits on a Penny? - California State University
advertisement

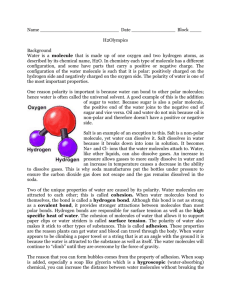
How Much Water Fits on a Penny?6 Students conduct an experiment to determine how many drops of water will fit on a penny and apply their knowledge of the properties of water and chemical bonds to explain the phenomenon. Suggested Grade Range: 7-9 Approximate Time: 1 hour State of California Standards: Science Content Standards Grades Nine through Twelve- Chemistry 2. Biological, chemical, and physical properties of matter result from the ability of atoms to form bonds from electrostatic forces between electrons and protons and between atoms and molecules. As a basis for understanding this concept: a. Students know atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds. b. Students know chemical bonds between atoms in molecules such as H2, CH4, NH3, HCCH, N, Cl, and many large biological molecules are covalent. Science Content Standards Grade Seven- Investigation and Experimentation 7.0 Scientific progress is made by asking meaningful questions and conducting careful investigations. As a basis for understanding this concept and addressing the content in the other three strands, students should develop their own questions and perform investigations. Students will: c. Communicate the logical connection among hypotheses, science concepts, tests conducted, data collected, and conclusions drawn from the scientific evidence. Mathematics Standards Grade 8: Probability and Statistics 10.0 Students know the definitions of the mean, median, and mode of distribution of data and can compute each of them in particular situations. Relevant National Standards: Mathematics Common Core State Standard: 7.SP 2. Use data from a random sample to draw inferences about a population with an unknown characteristic of interest. Generate multiple samples (or simulated samples) of the same size to gauge the variation in estimates or predictions. Gauge how far off the estimate or prediction might be. 6 An early version of this lesson was adapted and field-tested by Sherri Gonser, Karen Hardy, and David Macander, participants in the California State University, Long Beach Foundational Level Mathematics/General Science Credential Program. STEM Activities for Middle and High School Students How Much Water Fits on a Penny? 6-1 Next Generation Science Standards: MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. [Clarification Statement: Emphasis is on developing models of molecules that vary in complexity. Examples of simple molecules could include ammonia and methanol. Examples of extended structures could include sodium chloride or diamonds. Examples of molecular-level models could include drawings, 3D ball and stick structures or computer representations showing different molecules with different types of atoms.] [Assessment Boundary: Assessment does not include valence electrons and bonding energy, discussing the ionic nature of subunits of complex structures, or a complete depiction of all individual atoms in a complex molecule or extended structure.] Lesson Content Objectives: Practice using a pipette to be able to release one drop of liquid at a time. Be able to define surface tension, covalent bond, and cohesion. Conduct an experiment and analyze the data using central measures of tendency to determine how many drops of distilled water and soapy water will typically fit on a penny and compare the results. Be able to explain the phenomenon applying knowledge of the properties of water. Materials Needed: One pipette for each pair of students Two pennies for each pair of students One beaker of distilled water for each pair of students One beaker of soapy water for each pair of students Paper towels One copy per student of the “How Much Water Fits on a Penny?” activity sheet (included) Food coloring STEM Activities for Middle and High School Students How Much Water Fits on a Penny? 6-2 Summary of Lesson Sequence Introduce the lesson by allowing students to observe that drops of water can be added to a beaker already filled to the top without it spilling and by engaging students’ prior knowledge of the properties of water to explain what might be happening. Lead students through a discussion and note taking of the terms for this lesson: cohesion, surface tension, and covalent bond. Allow students to practice using a pipette before beginning their experiment. Guide students through their experiment to determine how many drops of distilled water will fit on a penny and how many drops of soapy water will fit on a penny. Check for students’ understanding by asking the provided key questions while students are experimenting. Close the lesson by allowing all the pairs of students to share their data to compile and analyze in a classroom discussion. Students work independently to summarize their understanding of the properties of water as they relate to this experiment by responding to comprehension questions on the “How Much Water Fits on a Penny?” activity sheet (included). Assumed Prior Knowledge Prior to this lesson students should already be familiar with the chemical properties of water and have an understanding of covalent bonds. Students should be able to calculate the mean of a data set and interpret the mean as a measure of central tendency of collected data. Classroom Set Up Each pair of students will need a pipette, a beaker of distilled water, and a beaker of soapy water to conduct the experiment. Paper towels should be available for each pair of students. To prevent students working with the pipettes and pennies prematurely, do not provide the pipettes or pennies until the guided practice portion of the lesson. Lesson Description Introduction Fill a beaker to the brim with distilled water colored with food coloring. Ask students: Is the beaker full? Because the beaker is filled to the brim, students may respond that it appears full. Begin to carefully add more water, one drop at a time, with a pipette. Ask students to count out loud how the number of drops added before the water begins to spill over the top. Engage students in a discussion connecting to their prior knowledge of the properties of water by asking: What chemical properties does water have? What have you learned about water that may help us understand why more water can be added to a full beaker without spilling over? STEM Activities for Middle and High School Students How Much Water Fits on a Penny? 6-3 Inform students: Today you will conduct an experiment and apply your knowledge of the chemical properties of water to understand its cohesive behavior. Before the experiment, we will review a few key concepts and you will practice using a pipette. Input and Model Lead students in their note taking by providing them with the key terms for the lesson: Cohesion: Water molecules are attracted to other water molecules. The oxygen end of water has a negative charge and the hydrogen end has a positive charge. The hydrogen atoms of one water molecule are attracted to the oxygen end of another water molecule. This attractive force is what gives water its cohesive property. Ask students: How could you draw a picture depicting the cohesive nature of several water molecules? What might be another, non-technical word for cohesion? If water molecules “stick” together, how can water be a liquid and not a solid? [The attraction that causes cohesion is different from the bonds that form to make a solid.] Surface tension: the cohesion of water molecules at the surface of a body of water. Covalent bond: a chemical bond that involves sharing a pair of electrons between atoms in a molecule. Guide Students Through Their Practice Provide pairs of students with a pipette with which to practice making single drops. Guide students by moving around the room and checking each student can carefully make one drop at a time onto a paper towel. If necessary, have students further practice by forming their initials with single drops of water onto a paper towel. The goal is for students to create similar sized drops of water each time. When each student has had practice using the pipette correctly, provide each pair with two pennies. Pairs of students will drop distilled water on one penny and soapy water on the other. Check for students’ understanding while they begin their experiment by asking them to predict: Do you think the number of drops of distilled water will be the same as the soapy water? How will you know when no more drops fit on the penny? How can you prevent water splashing off the penny? Provide each student with the “How Much Water Fits on a Penny?” activity sheet. Guide students through their experiment by giving them the following directions: Each student will have a chance to drop distilled water on one penny and drop soapy water on the other penny. STEM Activities for Middle and High School Students How Much Water Fits on a Penny? 6-4 When one student is dropping the water, the other student should be watching, counting out loud the number of drops, and recording the number of drops before the water spills over the edge of the penny. When a drop causes the water to spill over the edge of the penny, stop! Do this with the distilled water on one penny, then soapy water on the other penny. Dry off your pennies and switch roles with your partner so that your team can get data from multiple trials. It is important that each partner performs the experiment with both the distilled water and the soapy water because they may create different sized drops. We want to discover how many drops of water will fit on a penny and be able to explain what happens using our understanding of the properties of water. Move around the room to ensure students are using the pipettes carefully and counting the number of drops accurately. Checking for Understanding When everyone has had the opportunity to record their results, allow each pair of students to share their data with the rest of the class. Every student should record all of the data on his/her activity sheet (although they will be working in pairs, each students will be responsible for completing the activity sheet). The class should then calculate the average number of drops of distilled water and soapy water that will fit on a penny. During a whole class debrief, lead a discussion with the following questions: What causes the water to stay on the penny? What did you notice about the number of drops of soapy water versus the number of drops of distilled water? Based on the class data averages, what happens to the cohesion between water molecules when soap is added to water? (Teacher note: Soap causes the cohesiveness of the water molecules to decrease so they are not as strongly attracted to each other. Because of this, when soap is added to the water the number of drops that can be placed on the penny will decrease. The water molecules can't 'stick' together as well, so the water on top of the penny spills off sooner than it would with non-soapy water.) What are some reasons students might not get the exact same number of drops? Independent Practice Students should work independently to summarize their understanding of the properties of water as they relate to this experiment by analyzing the collected data and responding to comprehension questions on the “How Much Water Fits on a Penny?” activity sheet. Suggestions for Differentiation and Extension To extend this activity, students may explore the cohesiveness of other liquids, such as rubbing alcohol or vegetable oil, on their own and report their results to class. STEM Activities for Middle and High School Students How Much Water Fits on a Penny? 6-5 How Much Water Fits on a Penny? 1. Complete the chart with your data, your partner’s data and then data from the rest of the class. Team # 1 Partner 1 Partner 2 2 Partner 1 Partner 2 3 Partner 1 Partner 2 4 Partner 1 Partner 2 5 Partner 1 Partner 2 6 Partner 1 Partner 2 7 Partner 1 Partner 2 8 Partner 1 Partner 2 9 Partner 1 Partner 2 10 Partner 1 Partner 2 11 Partner 1 Partner 2 12 Partner 1 Partner 2 13 Partner 1 Partner 2 14 Partner 1 Partner 2 15 Partner 1 Partner 2 Drops of Distilled Water Drops of Soapy Water STEM Activities for Middle and High School Students How Much Water Fits on a Penny? 6-6 2. Calculate the mean number of drops for the class for distilled water to complete your chart. Calculate the mean number of drops for the class for soapy water to complete your chart. Use your understanding of the properties of water and the experimental data to respond to the following: 3. Draw a diagram several water molecules in distilled water showing how they might interact. Use arrows in your diagram and words such as: oxygen, hydrogen, bond, and attraction. 4. Describe what caused several drops of water to stay on the penny using the concepts and vocabulary you learned for this lesson. Why do you think the results were different for distilled and for soapy water? STEM Activities for Middle and High School Students How Much Water Fits on a Penny? 6-7