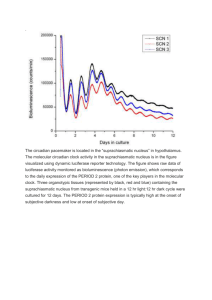
Photoreceptors in Arabidopsis thaliana: light perception, signal
advertisement

Planta (2002) 216: 1–16 DOI 10.1007/s00425-002-0831-4 R EV IE W Christian Fankhauser Æ Dorothee Staiger Photoreceptors in Arabidopsis thaliana : light perception, signal transduction and entrainment of the endogenous clock Received: 2 May 2002 / Accepted: 4 June 2002 Ó Springer-Verlag 2002 Abstract To keep track of fluctuations in spectral composition and intensity of incoming sunlight, plants engage a plethora of photosensory pigments. Absorption of light by these photoreceptors sets in motion signaling cascades that ultimately influence the plant’s physiology. Many light-controlled processes are based on modulation of gene activity in response to changes in irradiation. The molecular basis of this regulation and the downstream components transducing signals from the photoreceptors are not fully understood yet, but recent evidence suggests that some of those routes are rather short. The phytochrome photoreceptors have been found to influence light-responsive promoters by direct contact with transcription factors. Additionally, the cryptochrome blue-light receptors directly interact with a key repressor of photomorphogenesis, suggesting that light activation of photoreceptors could initiate photomorphogenesis through posttranslational regulation. This review focuses on recent insights into photosensory transduction mechanisms as well as on our current understanding of light entrainment of the endogenous clock. Keywords Arabidopsis Æ Circadian rhythm Æ Cryptochrome Æ Endogenous clock Æ Photoreceptor Æ Phytochrome Dedicated to Prof. Nikolaus Amrhein, Zürich, on the occasion of his 60th birthday. C. Fankhauser Department of Molecular Biology, 30 quai E. Ansermet, 1211 Genève 4, Switzerland D. Staiger (&) Institute for Plant Sciences, ETH, 8092 Zürich, Switzerland E-mail: dorothee.staiger@ipw.biol.ethz.ch Fax: +41-1-6321081 Introduction During their entire life cycle plants are very sensitive to their light environment. Light is a key factor influencing all major developmental transitions such as seed germination or flowering, for example. Plants accurately perceive fluctuations in the intensity, spectral quality, directionality, and periodicity (day length) of the incoming light. More than 80 years ago Garner and Allard (1920) demonstrated that the pigments necessary for such photomorphogenic responses were distinct from the pigments required for photosynthesis. These early photobiological experiments led to the discovery of the phytochromes, the first plant photoreceptors to be identified. Over the next few decades it became apparent that plants possess photoreceptors monitoring: UVB, UVA, blue, red and far-red light (Kendrick and Kronenberg 1994). The molecular nature of the UVB photoreceptors is still unknown, but three families of plant photoreceptors have now been identified: the phototropins, cryptochromes and phytochromes (Cashmore et al. 1999; Casal 2000; Christie and Briggs 2001; Nagy and Schaefer 2002; Quail 2002a, b). Upon light perception these photoreceptors initiate signaling cascades. Early steps in these processes are probably specific to a given photoreceptor, but there is clearly interaction and integration of the signals generated by the different photosensory pigments (Casal 2000; Quail 2002a). These same light signaling events also serve as a resetting cue for the circadian clock and there is increasing evidence suggesting that the light input pathways to the circadian clock and general light perception are closely linked (Devlin and Kay 2001). It is, however, premature to exclude the possible existence of a photosensory system dedicated to the circadian clock. We will focus on recent data concerning light perception in Arabidopsis as well as signal transduction during photomorphogenesis and resetting of the circadian clock by light. For specific details we suggest that the following reviews be consulted (Cashmore et al. 1999; 2 Fankhauser 2000; Hudson 2000; Lin 2000b; Smith 2000; Christie and Briggs 2001; Devlin and Kay 2001; McWatters et al. 2001; Roden and Carré 2001; Parks et al. 2001; Nagy and Schaefer 2002; Quail 2002a, b). Photoreceptors Phototropins The response of plants to unidirectional light had already been described by Charles Darwin and the first action spectrum was recorded by Julius von Sachs (Darwin 1881; Briggs and Huala 1999). Light-driven tropic growth is primarily induced by the blue region of the visible spectrum. The model plant Arabidopsis thaliana possesses two photoreceptors for this specific light response: phot1, formerly known as nph1 (non-phototropic hypocotyl 1), and phot2 (formerly npl1, NPH-1 like 1; Briggs et al. 2001). Phot1 and phot2 code for Ser/ Thr protein kinases with amino-terminal chromophorebinding domains. Two FMN molecules bind to so-called LOV (light, oxygen, voltage) domains. LOV domains are a subset of PAS (Per/Arnt/Sim) domains that are often found in proteins that sense environmental stimuli. Phot1 is associated with the plasma membrane, whereas the localization of phot2 is currently not known (Christie and Briggs 2001). We now know that in addition to their function in phototropic hypocotyl growth phot1 and phot2 are also essential for other blue-light responses. Their role has been demonstrated for the inhibition of hypocotyl growth during the very first minutes of blue light irradiation, for light-regulated ion fluxes, for chloroplast movement and for stomata opening (Folta and Spalding 2001a; Jarillo et al. 2001a; Kagawa et al. 2001; Kinoshita et al. 2001; Sakai et al. 2001; Babourina et al. 2002 ). Interestingly, the requirement for these two photoreceptors depends on the light intensity and the physiological response. For stomatal opening they have redundant functions whereas for positive phototropism of the hypocotyl phot1 clearly plays the predominant role in low light intensities, and both phototropins have a redundant function to detect high light intensities (Kinoshita et al. 2001; Sakai et al. 2001). Since phot1 and phot2 are light-regulated protein kinases, we have some clear ideas about the early steps in this signaling cascade (Christie et al. 1998; Sakai et al. 2001). Nuclear magnetic resonance (NMR) studies suggest that light induces a conformational change in the photoreceptor that modulates the protein kinase activity (Salomon et al. 2001). Genetic studies have identified a few additional elements important for phot1-mediated signaling (Motchoulski and Liscum 1999; Harper et al. 2000; Sakai et al. 2000). Our current knowledge indicates that phot1/phot2 are responsible for a number of specific blue-light responses for which the other photoreceptors are dispensable (Christie and Briggs 2001). In contrast, other light responses such as de-etiolation, transition to flowering or photic entrainment of the circadian clock require the cryptochromes and the phytochromes but not the phototropins (Casal 2000; Devlin and Kay 2001; Quail 2002b). It would, however, be premature to conclude that there is no interaction between the phototropins and the other photoreceptors (Folta and Spalding 2001a). Phototropism is not induced by red light, but a red light pretreatment enhances the effect of a subsequent blue light treatment. This enhancement of the phototropic curvature is mediated by the phytochromes, clearly suggesting a possible interaction between the two families of photoreceptors (Stowe-Evans et al. 2001). Cryptochromes The first blue-light photoreceptor to be identified at the molecular level was a cryptochrome (Ahmad and Cashmore 1993). Cryptochromes are completely unrelated in structure to phototropins; they are composed of an amino-terminal domain with homology to DNA photolyases and a carboxy-terminal domain of unknown biochemical function. This family of photoreceptors has probably evolved from an ancestral DNA photolyase, but has lost the photolyase activity; however, cryptochromes like DNA photolyases still bind to two chromophores, a Pterin and FAD (Cashmore et al. 1999; Christie and Briggs 2001; Lin 2000a). In Arabidopsis there are two cryptochromes, cry1 and cry2. Their carboxy terminal domains are highly divergent, but appear to have related functions (Guo et al. 1998; Yang et al. 2000). Since their discovery in Arabidopsis, cryptochromes have been encountered in numerous other plant species and also in animals (Cashmore et al. 1999). In animals they play a clear function in the circadian system, either as a photoreceptor to entrain the central oscillator or as a component of the oscillator itself, depending on the species (Stanewsky et al. 1998; van der Horst et al. 1999; Young and Kay 2001). Arabidopsis cryptochromes have important functions during de-etiolation and for the transition from vegetative to reproductive growth (Batschauer 1999; Cashmore et al. 1999; Lin 2000a; Quail 2002b). During de-etiolation, cry2 is particularly important in response to low blue light intensities in contrast to cry1, which has a prevalent role in response to strong blue light (Lin 2000b). Interestingly, in contrast to cry2, which is strongly down-regulated in response to blue light, cry1 protein levels are not lightregulated. This presumably explains why cry2 plays a major role in low light and cry1 in high light (Cashmore et al. 1999; Lin 2000a; Quail 2002b). Genome-wide analysis of light-regulated gene expression has demonstrated that about 15% of all transcripts are light-modulated (Ma et al. 2001; Tepperman et al. 2001). Interestingly, the subsets of genes regulated by different monochromatic lights appear to be very similar, but not identical (Kuno and Furuya 2000; 3 Ma et al. 2001). Such studies have also highlighted the importance of cry1 and cry2 in blue-light-modulated gene expression. It must, however, be pointed out that cry1cry2 double mutants still display blue-light-regulated gene expression, reinforcing the notion of co-action of multiple photoreceptors in blue light (Casal 2000; Ma et al. 2001). Cryptochromes are found in the nucleus of Arabidopsis plants, both in the light and in the dark (Cashmore et al. 1999; Kleiner et al. 1999). However a fusion protein between b-glucuronidase (GUS) and the carboxyterminal domain of CRY1 is cytosolic in the light and nuclear in the dark, raising the possibility for action in both sub-cellular compartments (Yang et al. 2000). Recent experiments strongly suggest that light activation exposes the carboxy-terminal portion of the protein, which then triggers the light-response (Yang et al. 2000). This de-etiolation response appears to require the physical interaction between the carboxy-terminal domain of the cryptochromes and the COP1 protein (Wang et al. 2001; Yang et al. 2001). COP1 was identified in a mutant displaying constitutive photomorphogenesis or de-etiolation (Deng et al. 1992). The recessive nature of cop1 alleles suggests that COP1 acts as a repressor of photomorphogenesis in the dark (Osterlund et al. 1999). One of the modes of action of COP1 is to inhibit an activator of light-regulated genes, HY5, presumably by targeting it to the proteasome (Osterlund et al. 2000). The direct interaction between COP1 and the cryptochromes suggests that upon light activation their carboxy terminus inhibits COP1 function. This may in turn allow HY5 to accumulate and promote de-etiolation. In keeping with such a model of a short signaling route downstream of the photoreceptors, only a limited number of mutants have been described that affect deetiolation in blue light specifically. The SUB1 (short under blue light) locus is clearly necessary for normal deetiolation in blue light; however, its role is not restricted to blue light since sub1 seedlings also show altered deetiolation in far-red light (Guo et al. 2001). SUB1 is a Ca2+-binding protein mainly localized in the cytoplasm. We currently do not know by what mechanism SUB1 modulates phytochrome and cryptochrome signaling (Guo et al. 2001). However, genetic data indicate that SUB1 also inhibits the bZIP transcription factor HY5 that works downstream of both the phytochromes and the cryptochromes (Guo et al. 2001). Phytochromes Phytochromes were originally defined as the photoreceptors mediating physiological responses that are induced by red light and, most importantly, that can be inhibited if a pulse of far-red light follows the inductive red light pulse (Kendrick and Kronenberg 1994). Based on these photobiological assays it was postulated that this photoreceptor must exist in two spectrally interchangeable forms: an inactive red-light-absorbing conformation (Pr) and an active far-red light-absorbing form (Pfr). This prediction was confirmed in 1959 when phytochrome was purified from etiolated oat seedlings by Butler and colleagues (Butler et al. 1959). We now distinguish between different modes of phytochrome-mediated light perception: low-fluence responses (LFRs) which obey the above description (red/ far-red reversibility), very low-fluence responses (VLFRs) which are most sensitive to red light, but also occur in response to a broad light spectrum and are not reversible, and high-irradiance responses (HIRs) which require prolonged illumination (or light pulses with a high frequency) (Shinomura et al. 2000; Smith 2000; Nagy and Schaefer 2002; Quail 2002b). In higher plants, phytochromes are encoded by a small gene family, designated PHYA to PHYE in Arabidopsis (Quail 2002b). In addition to the genes encoding the apoproteins, HY1 and HY2 are required to synthesize the chromophore that is covalently attached to the phytochrome (Davis et al. 1999; Muramoto et al. 1999; Kohchi et al. 2001). These light sensors are classified into type-I, or light labile, and type-II, or light stable, phytochromes (Quail 2002b). In Arabidopsis, phyA is the only type-I phytochrome. Genetic studies have indicated that phyA is responsible for the VLFR and the FR-HIR (Quail 2002b). Mutant analysis has shown that among the typeII phytochromes, phyB plays a predominant role (Quail 2002b). Phenotypes for single phyD and phyE mutants are generally only uncovered in double mutant combinations (Whitelam and Devlin 1997; Quail 2002b). Despite the apparently distinct modes of action of type-I and type-II phytochromes there are also a number of well-documented cases where they act additively, synergistically and sometimes even antagonistically (Casal 2000). A detailed list of all the light responses mediated by this family of photoreceptors is beyond the scope of this review. As an example, Fig. 1 illustrates the phenotypes of a phyB mutant during de-etiolation, vegetative growth and the transition to flowering. The most striking phenotypes of this mutant are the reduced inhibition of hypocotyl elongation by light, the reduced chlorophyll accumulation, the longer petioles and the early transition to flowering. Phytochromes are synthesized in their inactive Pr form in the dark. This light sensor is composed of two major domains separated by a small hinge region. The amino-terminal portion has the photosensory function and binds to the linear tetrapyrrole chromophore. Upon Pr-to-Pfr phototransformation, large structural changes occur in the protein (Quail 2002b). Those changes are postulated to activate the carboxy-terminal domain, which is composed of a PAS-related domain and a histidine-kinase-related domain. The exact nature of this ‘‘activation’’ is still under intense scrutiny but a lot of progress has been made on this topic recently. We now know that upon light perception phytochromes translocate from the cytosol to the nucleus (Nagy and Schaefer 2000, 2002). The subcellular 4 Fig. 1. Phenotypes of Columbia wild type and phyB mutants of Arabidopsis thaliana (Reed et al. 1993) throughout the life cycle. Left Wild-type seedlings and phyB-9 mutants grown for 6 days in white light. Right, top Wild type and phyB-9 mutants grown for 20 short days (8 h white light per day). Right, bottom After 5 weeks in short days, wild-type plants remain vegetative, whereas phyB-9 mutants have undergone floral transition localization of phytochrome had long been a subject of controversy. In fact, an effect of light on the subcellular distribution of phytochrome had already been reported in 1975 (Mackenzie et al. 1975), but these findings were largely ignored until this issue was conclusively settled in 1996 (Sakamoto and Nagatani 1996). In the nucleus phytochrome is found in discrete speckles. The functional significance of this re-localization is still not fully understood, but the very good correlation between the light requirements for nuclear translocation and defined phytochrome-mediated responses clearly indicates that it is an important regulatory step (Nagy and Schaefer 2000, 2002). We should, however, not conclude that only nuclear phytochrome has a function (Mustilli and Bowler 1997; Nagy and Schaefer 2002). A second important breakthrough in the field was the discovery of prokaryotic phytochromes (Vierstra and Davis 2000). Some of these photoreceptors have been studied in quite some detail and an important conclusion that can be drawn is that bacteriophytochromes are light-regulated histidine kinases (Yeh et al. 1997; Vierstra and Davis 2000; Bhoo et al. 2001). It is therefore clear that plant phytochromes are descendants of a bacterial light sensor that translates the light signal into a protein kinase activity. At this point it is worth pointing out that phototropins are also light-modulated protein kinases. This finding revived an old theory according to which plant phytochromes could be lightregulated enzymes. In fact there is good evidence that oat phytochrome A is a light-regulated Ser/Thr kinase (Yeh and Lagarias 1998; Fankhauser 2000). This might sound surprising since Ser/Thr kinases and His kinases belong to different protein families, and plant phytochromes posses a histidine-kinase-related domain rather than a Ser/Thr protein kinase domain. However, autophosphorylation activity has been demonstrated for oat phyA purified from both plant and recombinant sources (Yeh and Lagarias 1998; Fankhauser 2000). Interestingly, oat phytochrome A is phosphorylated in vivo and some of the mapped sites correspond to sites of in vitro autophosphorylation (Fankhauser 2000). Moreover, recombinant oat phyA also phosphorylates several substrates in vitro that might be relevant for photomorphogenesis in vivo (Ahmad et al. 1998; Fankhauser et al. 1999; Colon-Carmona et al. 2000). A recent publication suggests that this activity is actually important for phyA-mediated signaling in Arabidopsis (Maloof et al. 2001). Phytochrome signaling A large number of mutants deficient in light perception during de-etiolation have been identified since the pioneering screen of Maarten Koornneef in 1980 (Koornneef et al. 1980). We will not present a detailed description of all the identified mutants but try to highlight a few trends that emerge from these studies. 5 The mutants have been classified according to the color of light they are unable to perceive: blue, red and/ or far-red (Quail 2002a). The major photoreceptors sensing these light qualities during de-etiolation are the cryptochromes, phyB and phyA, respectively, and the signaling mutants are classified into pathways triggered by a particular photoreceptor (Quail 2002b). As anticipated from photobiological studies, some loci act specifically downstream of a single photoreceptor whereas others presumably integrate information from multiple light sensors (Casal 2000; Quail 2002a). Light does not only affect de-etiolation of seedlings. A number of more challenging screens using, for example, the shade-avoidance response or the transition to flowering in adult plants, have also uncovered components of these light-perception pathways (Devlin et al. 1998; Guo et al. 1998; Fowler et al. 1999; Park et al. 1999). We have currently very little understanding of the order of action of these numerous components. The analysis of a number of mutants suggests early branching in these signaling cascades so that it is probably much more accurate to describe them as signaling webs (Casal 2000; Quail 2002b; Wang and Deng 2002; Zhou et al. 2002). However, the analysis of mutants with a deetiolation (det) or constitutive photomorphogenic (cop) phenotype and mutants with reduced light sensitivity over the whole light spectrum suggests that all these branches finally converge (Quail 2002b). This conclusion is very much in line with global gene expression analysis revealing that the sets of genes regulated by different light qualities largely overlap (Ma et al. 2001). The molecular identification of numerous phytochrome-signaling components suggests that there are cytosolic, nuclear and actually also chloroplastic events (Moller et al. 2001; Quail 2002b). Yeast two-hybrid assays have identified several proteins that interact directly with the photoreceptor (Ni et al. 1998; Choi et al. 1999; Fankhauser et al. 1999; Jarillo et al. 2001b; Sweere et al. 2001). The importance of these proteins in phytochrome-mediated signaling has been confirmed using reverse genetic approaches. The best-understood part of phytochrome signaling is the ‘‘nuclear branch’’. The first identified component of this pathway is PIF3 (phytochrome interacting factor), a predicted transcription factor with a bHLH (basic helix-loop-helix) domain (Ni et al. 1998). Notably, PIF3 binds to phytochrome in a conformation-specific manner with Pfr having much greater affinity than Pr (Ni et al. 1999). Genetic and molecular data suggest that PIF3 is primarily involved in phytochrome B signaling (Ni et al. 1998; Halliday et al. 1999; Zhu et al. 2000). In vitro, phyB in its Pfr form makes a complex with PIF3 when it is bound to its DNA target site (Martinez-Garcia et al. 2000). Indeed, redlight induction of CCA1 (circadian clock associated) and LHY (late elongated hypocotyl) genes is attenuated in antisense plants with reduced PIF3 levels (MartinezGarcia et al. 2000). Taken together, these data establish a very short signaling cascade through which phytochromes following their light-triggered translocation into the nucleus influence gene activity by direct contacts with transcription factors already bound to their cognate target sequence. Remarkably, a direct effect of phytochrome on transcription was already proposed in 1987 when it was discovered that purified oat phyA augments the in vitro transcription rate of CAB genes in isolated barley nuclei (Mösinger et al. 1987). Genome-based expression studies have indicated that a large fraction of genes that are rapidly light induced in a phyA-dependent manner encode transcription factors (Tepperman et al. 2001). This suggests a mechanism by which phytochromes directly activate key transcriptional regulators such as PIF3, which subsequently upregulate a second wave of transcription factors such as CCA1 and LHY (Quail 2002b). It is very unlikely that PIF3 is the only transcription factor in the first class for several reasons. First of all, plants with reduced PIF3 levels have modest phenotypes compared with photoreceptor mutants. It must, however, be said that the phenotype of a true loss-of-function pif3 allele has not yet been reported. Moreover, another bHLH protein with significant similarity to PIF3 was genetically identified as a phyA-signaling component; however, RSF1/ HFR1/REP1 does not appear to physically interact with phyA (Fairchild et al. 2000; Soh et al. 2000; Spiegelman et al. 2000). In conclusion, these studies raise a number of important questions: How does phytochrome promote the transcriptional activity of PIF3? Are there many other transcription factors with similar roles to PIF3? Does this nuclear branch explain the whole story? The answer to this last question is most probably no. One could imagine that the cytosolic and chloroplastic events are just modulators of the really important nuclear branch. However, there are phytochrome responses that only require minutes and are therefore unlikely to require de novo protein synthesis (Folta and Spalding 2001b). Moreover, it is quite striking to compare the relatively strong morphological phenotype of phyB mutants (Fig. 1) with the considerably more modest impact of this photoreceptor on light-regulated gene expression (Ma et al. 2001). These data suggest additional phyB functions that do not require transcriptional regulation. The identification of PIF3 as a central regulator also implies a direct signaling route from phytochrome to the endogenous clock system, since two of its immediate targets, CCA1 and LHY, are important constituents of this system (see below). The endogenous clock Plants as photosynthetic organisms are particularly dependent on sunlight which is restricted to a limited time window of the day–night cycle. Thus, a day in the life of a plant resembles a day in an industrial enterprise in some aspects: exact timing of individual processes is required to optimally deploy limited resources and to coordinate the overall process in the most efficient way. 6 Like workers that obey a strict schedule, plants time their physiology and behavior. To do so, they have evolved an internal timekeeper, the endogenous clock. It allows the anticipation of regular fluctuations in the availability of the most important resource to plants, sunlight. The clock imposes a 24-hour rhythm on certain physiological processes so that they always occur at the optimal phase of the light–dark cycle. These ‘‘circadian’’ rhythms are the external manifestation of the internal clock, the so-called output. They range from leaf movement, growth processes, flower opening and fragrance emission to photosynthesis and carbon metabolism (Somers 1999; Staiger and Heintzen 1999; Barak et al. 2000; Harmer et al. 2001; McClung 2001). Underlying many of these physiological rhythms are endogenous rhythms in gene activity. They were first discovered for light-induced transcripts that continued to cycle between low and high levels even in continuous illumination (Kloppstech 1985; Nagy et al. 1988). Regulation is mostly exerted at the transcriptional level through clock-response elements in the promoters and cognate trans-acting factors (Carré and Kay 1995; Nagy et al. 1988; Wang and Tobin 1998; Staiger and Apel 1999; Staiger et al. 1999). A global view of endogenous rhythms in transcript abundance has been obtained through microarray analysis with probes sampled around the clock (Harmer et al. 2000; Schaffer et al. 2001). Clusters of genes operating in defined metabolic pathways, developmental processes and light responses have been identified that peak at similar times of the day. Among those, the well-studied phenylpropanoid pathway turned out to be under clock control. Twenty-three genes encoding biosynthetic enzymes are coordinately activated at the end of the night. This has been suggested to serve the production of UVprotective compounds in anticipation of dawn (Harmer et al. 2000). Notably, the very same screen uncovered circadian regulation of an MYB-type factor, PAP1 (production of anthocyanin pigment), whose overexpression causes activation of phenylpropanoid biosynthetic genes and enhanced accumulation of lignin, hydroxycinnamic acid esters, and flavonoids (Borevitz et al. 2000; Harmer et al. 2000). Clock-regulation of this key transcription factor thus may serve to synchronously activate the entire phenylpropanoid pathway. A similar microarray analysis of circadian gene expression in Drosophila melanogaster identified more than 100 cycling genes, many of which, surprisingly, map to the same chromosomal region and appear to be transcriptionally co-regulated (McDonald and Rosbash 2001; Ueda et al. 2002). Components of the clock system The persistence of circadian rhythms even in the absence of environmental timing cues indicates that they are driven by a self-sustaining oscillator. Integral to the oscillator are clock proteins that oscillate with a 24-h rhythm: clock genes are rhythmically transcribed and after a certain delay clock proteins feed back to inhibit transcriptional activity of their own genes (Dunlap 1999; Allada et al. 2001; Young and Kay 2001). Additionally, posttranscriptional regulation of the clock transcripts as well as posttranslational modifications of the clock proteins contribute to the maintenance of the 24-h periodicity (Edery 1999; Allada et al. 2001). The rhythmically produced clock proteins in turn translate temporal information into rhythmic physiology via output-signal transduction chains (Brown and Schibler 1999). Thus, the phases of clock gene mRNA and clock protein oscillations are indicators of internal time. While this picture has been firmly established for D. melanogaster, mammals and Neurospora crassa, the players in higher plants have not been unambiguously identified. Three proteins are candidates to form part of the central clock mechanism in A. thaliana (Yanovsky and Kay 2001). TOC1 (timing of CAB expression) has been identified by the Kay laboratory in a screen for mutants with aberrant output rhythms by monitoring the noninvasive luciferase (luc) marker under control of the cycling CAB2 promoter (Millar et al. 1995b). The toc1 mutation shortens the period of several rhythms, including gene expression, leaf movement and stomatal movement, and influences photoperiodic flower induction (Somers et al. 1998b). TOC1 is a nuclear protein with an N-terminal receiver domain known from response regulators of the His-to-Asp phosphorelay system (Strayer et al. 2000). Indeed, TOC1 has also been identified in a search for response regulators. Since it lacks two phospho-accepting aspartate residues, it presumably does not function via a classical phosphorelay mechanism. Accordingly it has been designated APRR1, Arabidopsis pseudo response regulator (Matsushika et al. 2000). Furthermore, two transcription factors with a single MYB-like domain, CCA1 and LHY, have been implicated in the generation of rhythms, as their constitutive overexpression disrupts several rhythms, including leaf movement and transcript oscillations (Schaffer et al. 1998; Wang and Tobin 1998). CCA1 overexpression also strongly represses the endogenous CCA1 transcript oscillations. Thus the CCA1 protein and CCA1 transcript are part of a negative autoregulatory circuit. Moreover, CCA1 and LHY negatively regulate each other’s transcripts, indicative of close interconnection between the feedback loops (Wang and Tobin 1998; Fowler et al. 1999). Based on the identification of these autoregulatory circuits it can be deduced that transcriptional feedback loops also play an important role in rhythm genesis in higher plants, although the transcription factors do not share sequence homology with known clock components from mammals, flies and fungi. Lately, a model has emerged showing that reciprocal regulation between the CCA1/LHY loops on the one hand and TOC1 on the other hand may constitute the core of an oscillator in Arabidopsis (Alabadi et al. 2001). 7 Negative interaction between CCA1/LHY and TOC1 was inferred from the opposite phases of the TOC1 and CCA1/LHY mRNAs that cycle with an evening peak and a peak around dawn, respectively (Strayer et al. 2000). Indeed, in plants constitutively producing LHY or CCA1, TOC1 mRNA oscillations are depressed to trough levels, implicating LHY and CCA1 as negative regulators of TOC1. In turn, LHY and CCA1 oscillations have a shorter period in toc1 mutant plants and peak levels are strongly reduced in the presumptive lossof-function mutant toc1-2, indicating a positive action of TOC1 on CCA1 and LHY (Alabadi et al. 2001). Since CCA1 and LHY are not arrhythmic in the absence of functional TOC1, one may envisage factors acting redundantly with TOC1 (see below). CCA1 was identified through its interaction with the promoter of the LHCB1*1 (CAB2) gene that cycles with a morning peak. CCA1 binds an AAA/CAATCT motif found within a 36-bp element mediating circadian regulation (Carré and Kay 1995; Wang and Tobin 1998). CCA1 and LHY also influence transcripts with an evening phase, as constitutive expression of CCA1 or LHY causes arrhythmic expression of AtGRP7/CCR2 encoding a glycine-rich RNA-binding protein that itself is part of a subordinated negative autoregulatory circuit (Heintzen et al. 1997; Schaffer et al. 1998; Wang and Tobin 1998). This regulation has been predicted to occur through putative CCA1-binding sites within a minimal clock response element of the AtGRP7/CCR2 promoter (Staiger and Apel 1999; Alabadi et al. 2001; Quail 2002b). An AAAATATCT element with strikingly similarity to the CCA1-binding site has been discovered in genes cycling with an evening phase, including TOC1, and CCA1 and LHY indeed bind to this element of the TOC1 promoter (Harmer et al. 2000; Alabadi et al. 2001). As TOC1 cycles in antiphase to CAB2, binding of CCA and LHY to the same element in either the TOC1 or CAB2 promoter has opposite effects on promoter activity. Thus, promoter architecture presumably plays an important role in determining the response to these regulators. Additional layers of complexity may have to be added to this outline, since CCA1 and LHY, as well as TOC1/APRR1, turned out to be members of multigene families (Matsushika et al. 2000). The ‘‘APRR1/TOC1 quintet’’ family is under co-ordinate clock control. APRR9 is induced by light and appears shortly after dawn. APRR7, APRR5, APRR3 and APRR1/TOC1 subsequently appear sequentially at 2- to 3-h intervals. Their free-running rhythms are differentially affected by overexpression of APRR1/TOC1 and CCA1. Moreover, light induction of APRR9 is absent in APRR1-ox plants. These observations suggest that in addition to the mutual interaction between CCA1 and TOC1/APRR1 each member of the ‘‘APRR1/TOC1 quintet’’ is under exquisite control of CCA1 and APRR1/TOC1, although the molecular underpinnings remain to be resolved (Makino et al. 2002; Matsushika et al. 2002). Furthermore, several CCA1 and LHY homologues have been identified that oscillate with a peak mostly around dawn and thus have been designated the Reveille (RVE) family (Andersson et al. 1999). This opens the possibility that homo- and heterotypic interactions within the families may contribute to the core oscillatory loop. Light input to the clock A prime function of the endogenous clock is to provide an endogenous estimate of the environmental time. In constant conditions in the laboratory, the endogenous clock runs with a period of about, but not exactly, 24 h whereas in nature, the period is exactly 24 h. In other words, to be in synchrony with the outside world, plants have to monitor the 24-h geophysical cycle and adjust their endogenous clock on a daily basis. Periodic changes between dawn and dusk – with light being the principle ‘‘zeitgeber’’ – serve to set the phase of the endogenous clock. As phase is determined by the level of a clock component, entrainment of the oscillator requires that a clock component reacts to incoming light in a manner dependent on the time of day. Recent data obtained in several laboratories begin to shed some light on the components of the Arabidopsis oscillator system that may be a target for this ‘‘entrainment’’, the photoreceptors that provide light information as well as the intermediates of the respective input signaling cascades. Photoreceptors The available Arabidopsis photoreceptor mutants have allowed their roles in light input to the clock to be tested. Broadly speaking, no single photoreceptor has been identified as having an essential function to reset the circadian clock. This was illustrated very clearly for a phyAphyBcry1cry2 quadruple mutant that is severely impaired for de-etiolation but retains leaf movement rhythms that can entrain to different photoperiods (Yanovsky et al. 2000a). The importance of those photoreceptors could, however, be uncovered by testing the period length of circadian-regulated transcripts during constant illumination with monochromatic light. In extended darkness, the free-running period of CAB2::LUC rhythms lengthens to 30–36 h, a much longer value than observed for free-running periods in other organisms (Millar et al. 1995a). The period becomes shorter at increasing light intensities. This inverse relationship between period length and light intensity in plants and light-active animals is known as Aschoff’s rule (Aschoff 1979). Both blue light and red light contribute to this period shortening (Millar et al. 1995a). Mutants deficient in specific photoreceptors revealed that phyA, phyB, phyD and phyE mediate red-light effects on the pace of the clock, 8 and cry1 and cry2 mediate blue light input (Somers et al. 1998a; Devlin and Kay 2000). cry1cry2 double mutants retain rhythmicity of CAB2::LUC expression, in contrast to mice that upon knockout of both cry1 and cry2 become completely arrhythmic (Somers et al. 1998a; van der Horst et al. 1999; Devlin and Kay 2000). Cryptochromes in plants are thus not integral components of the clockwork in contrast to their mammalian counterpart. Co-action between cry2 and phytochromes to control the pace of the clock is demonstrated in cry2 mutants that have a longer period of CAB2::LUC mostly in white light, indicating requirement for simultaneous activation of cry and phy (Mas et al. 2000). Indeed, cry2 physically interacts with phyB in discrete regions of the nucleus (Mas et al. 2000). Cry1 mutants show deficiency in entrainment of CAB2::LUC rhythms also in low-fluence-rate red light (Devlin and Kay 2000). As period lengthening in cry1phyA double mutants is similar to that in the individual mutants and cry1 does not significantly absorb red light, cry1 presumably acts as a signaling intermediate downstream of phyA. In fact, a physical interaction between phyA and cry1 has previously been demonstrated: cry1 undergoes light-dependent phosphorylation by phyA (Ahmad et al. 1998). Evidence for an impact of phyA and cry1 on circadian rhythms was also obtained through an intriguing observation made for the catalase 3 transcript that cycles with an evening peak. In prolonged darkness, the transcript attains a high constitutive level, in contrast to many oscillating transcripts in plants that rapidly damp to undetectable levels in constant darkness. This damping to high levels is phyA and cry1 dependent but the mechanism involved is so far not understood (Zhong et al. 1997). Mutants affected in phot1 show no defect in signaling to the clock (Devlin and Kay 2001). The role of phot2 has not yet been tested. Nevertheless, the repertoire of clock photoreceptive molecules may still expand. Good candidates are members of the ZEITLUPE/FKF/LKP family. The ztl mutant has a period-lengthening phenotype of multiple rhythms that is strongly dependent on light intensity, and in addition it has a late-flowering phenotype (Kiyosue and Wada 2000; Somers et al. 2000). Overexpression of LKP2 (LOV Kelch protein 2) causes arrhythmicity of transcript oscillations both in constant light and darkness, as well as delayed photoperiodic flower induction (Schultz et al. 2001). A third member, FKF1 (flavin-binding, kelch repeat, F box) was also identified on the basis of a late-flowering phenotype of the fkf1 null mutation (Nelson et al. 2000). Deletion of FKF1 influences the waveform of circadian oscillation and FKF1 itself cycles, in contrast to ZTL and LKP2. Although based on these observations it is difficult to assign an exact role to this trio, these proteins feature three interesting motifs suggestive of a possible biochemical function (Kiyosue and Wada 2000; Nelson et al. 2000; Somers et al. 2000; Schultz et al. 2001). By analogy with phototropins, their N-terminal LOV/PAS domain could bind a chromophore. However, the effects of ZTL on period length are observed both in red and blue light, suggesting that in red light ZTL might act as a signaling intermediate downstream of a phytochrome (a situation analogous to the cry1/phyA interaction in red light, see above). This suggests that the LOV/PAS domain may mediate protein–protein interaction and any light-dependent activity of ZTL may result, directly or indirectly, from stimulation by other photoreceptors. A recent study indeed shows interaction of ZTL/ADAGIO with both cry1 and phyB (Jarillo et al. 2001b). ZTL/ FKF1/LKP2 also have F-box motifs that in other proteins serve to target them for ubiquitin-dependent proteolysis, as well as kelch domains mediating protein– protein interaction. Based on the predicted functions of the domains, the ZTL family members may interact in a light-dependent manner with proteins affecting period length. Through targeting these molecules for proteolytic degradation, they may influence the length of the circadian cycle. Potential targets include clock-associated molecules, including ZTL itself, as well as photoreceptors. Expression of photoreceptors is under control of the clock A recent investigation shows that the genes encoding the major photoreceptors are not uniformly active throughout the day. Rather, the promoter activity of phytochromes and cryptochromes is diurnally regulated (Bognar et al. 1999; Toth et al. 2001). Transcripts encoding the light-stable proteins phyB, phyC, phyD, phyE and cry1 peak during the early hours of the daily light phase whereas those encoding light-labile phyA and cry2 reach their highest level close to dusk (Fig. 2). This may reflect the importance of the newly synthesized proteins, e.g. for detection of photoperiod extension and end-of-day response to far-red. The mRNA oscillations persist in constant light and in constant darkness, indicative of endogenous control. Thus, photoreceptors, on the one hand, transduce light signals to the clock and thus are part of the input pathway and, on the other hand, have to be considered part of the clock output. The rhythmically produced photoreceptors can, by feedback, temporally restrict light input to the clock so that resetting cues are most efficiently perceived at the appropriate times of day. Fig. 2. Time-of-day specific expression of Arabidopsis photoreceptors. Depicted is the relative phase of promoter activity as determined by measuring luciferase activity in transgenic Arabidopsis plants. zt Zeitgeber time, hours after lights on. Modified from Toth et al. (2001) 9 Reduced synthesis of photoreceptive molecules during the night may serve to buffer the circadian clock against moonlight, for example, which does not signal dawn or dusk and thus should not reset the clock (Bünning and Moser 1969). To exert this type of regulation, protein levels of the photoreceptors should also change systematically over the course of the day. This has so far only been demonstrated for CRY2 (Bognar et al. 1999; El-Din El-Assal et al. 2001). However, accumulation of phytochromes in the nucleus appears to vary over the course of the light– dark cycle. Moreover, extensive interactions both among photoreceptors and with signaling components provide a basis for additional layers of posttranslational control of activity that we are only beginning to appreciate (Nagy and Schaefer 2002; Quail 2002a, b). Light input transduction As described in the previous section, partially overlapping functions and extensive cross-talk between multiple photoreceptors are apparent for the light input to the clock and general photomorphogenesis. We still know little about the extent to which the corresponding downstream signaling cascades are shared. ELF3 (early flowering) is among the first components with a demonstrated role in light signaling to the clock. Elf3 mutants flower as early in short days as in long days. Leaf movement and LHCb1*1 (CAB2) promoter activity are arrhythmic in continuous light whereas rhythmicity is retained in continuous darkness, suggesting that the defect may affect light input (Hicks et al. 1996). In wild-type plants, phytochrome induction of CAB2 is rhythmically repressed by the clock, allowing a higher inducibility during the day than during the night (Millar and Kay 1996). This circadian ‘‘gating’’ of the acute light response is lost in elf3, leading to constitutive CAB activation (McWatters et al. 2000). Thus, the apparent arrhythmicity in constant light at least in part results from an aberrant reaction to light. In a complementary approach, ELF3 signaling to the clock was tested by assaying phase shifts elicited by light pulses administered at different times. Phase shifts were reduced in plants constitutively overproducing ELF3 during the night, again indicating that ELF3 then negatively modulates light input (Covington et al. 2001). Elf3 transcript and protein oscillate, reaching their highest concentration at dusk, i.e. around the time they are predicted to be required to antagonize light input to the clock (McWatters et al. 2000; Covington et al. 2001; Hicks et al. 2001; X.L. Liu et al. 2001). ELF3 has been proposed to be part of an additional loop through which it feeds back to periodically restrict light input to the clock (McWatters et al. 2000). Similar to the rhythmically produced photoreceptors that feed back onto light input (Fig. 3), feedback by an oscillating signaling intermediate Fig. 3. The Arabidopsis circadian system. Photoreceptors are under clock control and feed back to modulate light input to the clock allows rhythmic input to the oscillator under otherwise constant conditions. The exact role of ELF3 in general light signaling is not understood. The phenotype of elf3 mutants that is most apparent in red light suggests a role downstream of the phyB photoreceptor, and a physical interaction between phyB and ELF3 has been shown (Reed et al. 2000; X.L. Liu et al. 2001). However, double mutants between elf3 and phyB are additive under certain light conditions, clearly indicating that ELF3 does not exclusively work downstream of the phyB photoreceptor. A role in light signaling has also been demonstrated for GIGANTEA, originally identified in a mutant with extremely delayed onset of flowering in long days. The gi mutation subsequently was found to affect leaf movement rhythms and transcript oscillations (Fowler et al. 1999; Park et al. 1999). The period-shortening phenotype of the gi-1 allele is dependent on fluence rate, suggesting that GI mediates light input to the clock. On the other hand, GI itself undergoes circadian oscillation. Rhythmic expression of GI as well as of several other cyclic transcripts is impaired in gi-2 mutants that lack functional GI protein. Therefore, a model has been proposed according to which GI is part of a feedback loop that is required for a central oscillator to maintain period and amplitude (Fowler et al. 1999; Park et al. 1999). A further gi allele was recovered in a screen for defects in the inhibition of hypocotyl elongation in red light (Huq et al. 2000). GI therefore functions in phyBmediated photomorphogenesis and affects the light input to the clock. Other light signaling intermediates that are cyclically expressed include SPA1 and RPT2, but a potential function in clock regulation has not yet been reported (Harmer et al. 2000). Of the numerous other light signaling components operating downstream of the photoreceptors only very few have been tested for a direct role in light input to the clock. Cop1 and det1 mutants with a de-etiolated phenotype in the absence of light shorten the free-running period of the CAB2::LUC rhythm in the dark although the mechanism has not been investigated (Millar et al. 1995a). The action of both FHY1 (far-red elongated hypocotyl) and FHY3, two phyA signaling components, 10 is required for phase shifting of leaf movement rhythms in response to far-red light. This is interesting since FHY3 is only required for a subset of phyA-mediated responses and suggests that phyA in its HIR signaling mode is necessary for resetting the clock in response to far-red light (Yanovsky et al. 2000b, 2001). Oscillator components responding to light input Some of the clock components that are directly influenced by light have been identified. Whereas TOC1 mRNA is not directly light-responsive, CCA1 mRNA is rapidly and transiently induced by red light in etiolated seedlings (Wang and Tobin 1998). By analogy, rapid induction of CCA1 and LHY following phytochrome activation has been implicated in clock resetting by light at dawn: This presumably is mediated by the bHLH factor PIF3 through the same short transcriptional cascade as described above (Martinez-Garcia et al. 2000). Figure 4 summarizes a current model (Alabadi et al. 2001; Quail 2002b). Upon light activation, phytochrome migrates to the nucleus and forms complexes with PIF3 bound to the G box of CCA1 (and, by inference, LHY). Activation at dawn leads to increased CCA1 and LHY transcript and protein levels. Perturbation of CCA1 and LHY steady-state levels then affects TOC1 levels through reciprocal regulation, as LHY and CCA1 coordinately repress TOC1. Progressive decline in CCA1 and LHY levels over the course of the day subsequently allows TOC1 to accumulate which then, directly or Fig. 4. Model of how light input may influence a core oscillator in Arabidopsis. Red-light activation of phytochrome leads to translocation into the nucleus where Phy interacts with promoter-bound PIF3. This leads to rapid activation of CCA1 and, perhaps, LHY. LHY and CCA1 coordinately repress TOC1. Progressive decline in CCA1 and LHY levels in the course of the day allows TOC1 to accumulate which then, directly or indirectly, stimulates production of CCA1 and LHY. In addition to these interactions within the presumptive core oscillator, high CCA1 and LHY levels in the morning activate CAB2 and likely other morning genes, and may repress evening genes like AtGRP7/CCR2. Elevated TOC1 levels later in the day then allow expression of evening genes. Modified according to Alabadi et al. (2001) and Quail (2002b) indirectly, stimulates production of CCA1 and LHY. In addition to these interactions within the presumptive core oscillator, high CCA1 and LHY levels in the morning act downstream to activate CAB2 and likely other morning genes and possibly repress evening genes, for example AtGRP7/CCR2. Decreasing levels of CCA1/LHY and increasing levels of TOC1 later in the day then allow expression of evening genes. According to this model, PIF3 may be a key player, integrating phytochrome regulation of the circadian system as well as of photomorphogenesis. One should be aware that this may not yet be the complete picture. APRR1/TOC1 has been shown to associate with PIF3 as well as with a PIF3 like protein, PFL1 (Makino et al. 2002). If these interactions prove to occur in planta, they may add another twist. It remains to be determined whether CCA1 induction depends on time-of-day, a prerequisite for a stimulus that resets the clock. Furthermore, it needs to be established whether CCA1 and LHY would also serve as targets for clock resetting by blue or far-red light. As PIF3 has been implicated in phytochrome signaling specifically, at least blue light may be read by other components. Additional ‘‘zeitgebers’’ Although light represents the most prominent ‘‘zeitgeber’’ in higher plants, regular temperature changes can entrain the oscillator in the absence of periodic light cues. In mustard and Arabidopsis plants grown from seed in continuous light at a constant temperature, transcripts encoding the chlorophyll-binding proteins or glycine-rich RNA-binding proteins do not undergo circadian oscillations. If plants are exposed to regular temperature cycles, oscillations with a 24-h period are synchronized and/or initiated (Heintzen et al. 1994a, b; Somers et al. 1998b). In seedlings of Lycopersicum esculentum, temperature cycles are even dominant over light programs in the control of rhythms in polyamine content (N’Doye et al. 1994). At present the way in which plants perceive temperature cues is not understood. The large temperature step Arabidopsis plants encounter upon transfer from stratification conditions at 4 °C to regular growth conditions at ambient temperature does not reset the phase (Zhong et al. 1998). This has been taken to suggest that during an initial phase the oscillator is unable to respond to temperature. In contrast, imbibition of dried seeds can act to entrain circadian rhythms in Arabidopsis (Zhong et al. 1998). Photoperiodism The endogenous clock not only tells time of the day, but also provides an internal estimate of the season of the year. Resetting the clock by light enables plants to detect 11 the gradual shift in the time of sunrise during the seasonal progression. Monitoring the seasons offers a selective advantage to plants by allowing appropriate reactions in order to prevent damage by unfavorable environments. For example, at high latitudes, short days in autumn lead to induction of bud dormancy and cold hardiness in anticipation of adverse winters. Most importantly, the transition from vegetative to reproductive growth in many plant species is controlled by annual modulation of photoperiod and thermoperiod (Simpson and Dean 2002). This ensures that onset of flowering and seed set terminate in a favorable season. Short-day induction, for example, would allow plants to flower before a dense leaf canopy develops. In out-crossing species, coordination of flowering within the population by way of photoperiodic control increases the possibility for genetic recombination. In the 1930s, Erwin Bünning had suggested that plants rely on their endogenous timekeeper – the circadian clock – to measure daylength (Bünning 1936). Experimental support now comes from the discovery of mutants in the facultative long-day plant Arabidopsis that are disrupted in both circadian rhythmicity and flowering time: Lhy mutant plants with disrupted circadian rhythms flower late in long days and the lateflowering mutant gi additionally affects leaf movement rhythms and LHC transcript oscillations (Schaffer et al. 1998; Fowler et al. 1999; Park et al. 1999). The elf3 mutant is insensitive to daylength, flowering as early in short days as in long days, and affects light input to the circadian clock (Hicks et al. 1996; McWatters et al. 2000; Covington et al. 2001; X.L. Liu et al. 2001). Two related views of how clock function may translate into photoperiodic flower induction have been proposed (Fig. 5). According to the model of ‘‘external coincidence’’, environmental light impinging on the plant in a light-sensitive phase of an endogenous circadian rhythm would promote transition in flowering in long-day plants, for example Arabidopsis, and retard it in short-day plants, for example rice. The ‘‘internal Fig. 5. Model of photoperiodic control by rhythmically expressed flowering time genes. In short days (SD), high expression of a regulatory gene (such as CO) would be largely confined to the dark phase (solid line). In long days (LD), light would modify the rhythms such that high-level expression would occur concomitant with environmental light (external coincidence) and/or that highlevel expression of one regulator (solid line) would occur concomitantly with that of another regulator (dashed line; internal coincidence) coincidence’’ model, in contrast, posits that two co-existing endogenous rhythms would fall into phase under flower-promoting photoperiods (Wagner et al. 1998). CONSTANS (CO) is a flowering-time gene that promotes floral transition specifically in long days. It has recently been found that CO mRNA levels are under clock control (Samach et al. 2000; Suarez-Lopez et al. 2001). The observation that these oscillations are modified according to the photoperiod suggests that CO levels may be one of the endogenous rhythms postulated by these models: under short days, the CO peak is largely confined to darkness, whereas under inductive long days, high CO levels coincide with light (SuarezLopez et al. 2001). Furthermore, CO rhythms are affected in mutants with altered photoperiodic response: CO oscillates at a higher level in the early flowering elf3 mutant. In contrast, CO oscillates with lower amplitude in the late-flowering gi-3 mutant, and in the lhy mutant, CO is expressed at a reduced level with a different phase. The late-flowering phenotypes are corrected by CO overexpression. As LHY, GI and ELF3 all are part of the circadian system, the clock presumably influences the photoperiodic response by setting the CO rhythm. CO then promotes flowering via downstream genes (Samach et al. 2000). Indeed, it causes an immediate target, FT, to oscillate with a similar phase (SuarezLopez et al. 2001). A QTL (quantitative trait locus) controlling photoperiodic flowering in rice, a short-day plant, turned out to be related to CO, and a CO ortholog from another short-day plant, Pharbitis nil, corrects late flowering of the Arabidopsis co mutant (Yano et al. 2000; J. Liu et al. 2001). This raises the intriguing possibility that related proteins may participate in photoperiodic timekeeping in both long-day and short-day plants, although the exact mode of action remains to be resolved. Although a detailed analysis of CO protein levels under the different photoperiods has not yet been reported, initial experiments point to CO being unstable, providing an additional possibility for regulation of its activity by light (Suarez-Lopez et al. 2001). Interestingly, the steady-state abundance of the CRY2 blue-light receptor has been found to play a crucial role in determining floral transition. QTL analysis has identified cry2 as a trait distinguishing the flowering time behavior of two Arabidopsis ecotypes, demonstrating the importance of this locus for adaptation of Arabidopsis in the natural environment (El-Din El-Assal et al. 2001). cry2 mutants are late flowering in long days specifically, pointing to a role of this photoreceptor to detect daylength extension (Guo et al. 1998). Indeed, CRY2 levels may themselves be a marker for photoperiod: whereas in long days, CRY2 levels do not notably vary in the light, CRY2 is rapidly down-regulated in the light during short days and only re-accumulates at dusk. Moreover, a single amino acid substitution in CRY2 of the EDI accession leads to a substantial increase in CRY2 protein during short days and confers an early flowering phenotype. Rhythmicity of the CAB2::LUC marker and leaf 12 movement is not affected by this mutation, suggesting that the effect of cry2 on photoperiodic flowering may not be exerted via cry2 signaling through the clock but may rather affect flowering-time genes directly (El-Din El-Assal et al. 2001). Current efforts focus on unraveling the interaction between the multiple pathways mediating transition to flowering in response to light and photoperiod, to endogenous developmental cues, to gibberellins or vernalization. Interestingly, two of the key factors integrating floral pathways are immediate targets of CO (Samach et al. 2000; Simpson and Dean 2002). Conclusion Over recent years, more and more evidence has accumulated showing overlapping functions between individual photoreceptors during photomorphogenesis. Furthermore, in addition to transduction chains triggered specifically by one photoreceptor, extensive crosstalk between signaling cascades downstream of multiple photoreceptors has become apparent. Thus, it may be more appropriate to view the downstream events in the light of an entire signaling network (Casal 2000; Quail 2002a). Entrainment of the endogenous clock by environmental light appears to use, at least partly, the welldescribed photosensory pigments as well as many of the signaling intermediates also involved in photomorphogenetic regulation, pointing to extensive cross-talk. In addition, previously undescribed molecules have been found to play a prominent, although as yet not fully understood, role in light regulation of the circadian system, as for example the ZTL family. Also, phyD and phyE play only a minor role during de-etiolation but clearly contribute to entrainment of the clock (Aukerman et al. 1997; Devlin et al. 1998; Devlin and Kay 2000). Most of the photoreceptors, as well as some components of the light input pathway, are themselves under circadian control, suggesting that these components feed back to regulate the plant’s light responses in a time-of-day dependent manner. In summary, despite the fact that our current understanding is far from complete it appears that it will not be easy to tease apart the function of light perception from its specific role to entrain the circadian clock. Acknowledgements We would like to express our sincere gratitude to Nick Amrhein, a dedicated teacher and good colleague and mentor, for his help, support and encouragement over the years.Due to space constraints, we have attempted to highlight a few examples of recent progress in unraveling light signaling and have had to use reviews rather than original publications on a number of occasions. We apologize to those colleagues whose contributions could not be included. We thank Dr. Dieter Rubli and Nicolas Roggli for help with the figures. Work in our laboratories has been supported by the Swiss National Foundation (631-058151.99 for C.F. and 31-052475.97 for D.S.), the EMBO Young Investigator Program (C.F.) and the ETH Research Commission (D.S.). References Ahmad M, Cashmore AR (1993) HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366:162–166 Ahmad M, Jarillo J, Smirnova O, Cashmore AR (1998) The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol Cell 1:939–948 Alabadi D, Oyama T, Yanovsky MJ, Harmon FG, Mas P, Kay SA (2001) Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293:880–883 Allada R, Emery P, Takahashi JS, Rosbash M (2001) Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci 24:1091–1119 Andersson CR, Harmer SL, Schultz TF, Kay SA (1999) The Reveille (REV) family of DNA binding proteins and the circadian clock. In: Abstracts of the 10th international conference on Arabidopsis research, Melbourne, 4–8 July 1999. http:// arabidopsis.org/abstract_australia.pdf Aschoff J (1979) Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol 49:225–249 Aukerman M, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino R, Sharrock R (1997) A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9:1317–1326 Babourina O, Newman I, Shabala S (2002) Blue light-induced kinetics of H+ and Ca2+ fluxes in etiolated wild-type and phototropin-mutant Arabidopsis seedlings. Proc Natl Acad Sci USA 99:2433–2438 Barak S, Tobin EM, Green RM, Andronis C, Sugano S (2000) All in good time: the Arabidopsis circadian clock. Trends Plant Sci 5:517–522 Batschauer A (1999) Light perception in higher plants. Cell Mol Life Sci 55:153–166 Bhoo SH, Davis SJ, Walker J, Karniol B, Vierstra RD (2001) Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature 414:776–779 Bognar LK, Hall A, Adam E, Thain SC, Nagy F, Millar AJ (1999) The circadian clock controls the expression pattern of the circadian input photoreceptor, phytochrome B. Proc Natl Acad Sci USA 96:14652–14657 Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12:2383–2394 Briggs WR, Huala E (1999) Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol 15:33–62 Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A, Okada K, Salomon M, Rudiger W, Sakai T, Takano M, Wada M, Watson JC (2001) The phototropin family of photoreceptors. Plant Cell 13:993–997 Brown SA, Schibler U (1999) The ins and outs of circadian timekeeping. Curr Opin Genet Dev 9:588–594 Bünning E (1936) Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber Dtsch Bot Ges 54:590–607 Bünning E, Moser I (1969) Interference of moonlight with the photoperiodic measurement of time by plants, and their adaptive reaction. Proc Natl Acad Sci USA 62:1018–1022 Butler WL, Norris KH, Siegelman HW, Hendricks SB (1959) Detection, assay, and preliminary purification of the pigment controlling photoresponsive development of plants. Proc Natl Acad Sci USA 1703–1708 Carré I, Kay S (1995) Multiple DNA–protein interactions at a circadian-regulated promoter element. Plant Cell 7:3039–3051 Casal JJ (2000) Phytochromes, cryptochromes, phototropin: photoreceptor interactions in plants. Photochem Photobiol 71:1–11 Cashmore AR, Jarillo JA, Wu YJ, Liu D (1999) Cryptochromes: blue light receptors for plants and animals. Science 284:760–765 13 Choi G, Yi H, Lee J, Kwon YK, Soh MS, Shin B, Luka Z, Hahn TR, Song PS (1999) Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature 401:610–613 Christie JM, Briggs WR (2001) Blue light sensing in higher plants. J Biol Chem 276:11457–11460 Christie JM, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs WR (1998) Arabidopsis NPH1: a flavoprotein with the properties of a photoreceptor for phototropism. Science 282:1698–1701 Colon-Carmona A, Chen DL, Yeh KC, Abel S (2000) Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol 124:1728–1738 Covington MF, Panda S, Liu XL, Strayer CA, Wagner DR, Kay SA (2001) ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13:1305–1315 Darwin C (1981) The power of movement in plants, Da Capo press reprinted edn, 1996. Da Capo, New York Davis SJ, Kurepa J, Vierstra RD (1999) The Arabidopsis thaliana HY1 locus, required for phytochrome-chromophore biosynthesis, encodes a protein related to heme oxygenases. Proc Natl Acad Sci USA 96:6541–6546 Deng XW, Matsui M, Wei N, Wagner D, Chu AM, Feldmann KA, Quail PH (1992) COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell 71:791–801 Devlin PF, Kay SA (2000) Cryptochromes are required for phytochrome signaling to the circadian clock but not for rhythmicity. Plant Cell 12:2499–2510 Devlin PF, Kay SA (2001) Circadian photoperception. Annu Rev Physiol 63:677–694 Devlin PF, Patel SR, Whitelam GC (1998) Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10:1479–1487 Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96:271–290 Edery I (1999) Role of posttranscriptional regulation in circadian clocks: lessons from Drosophila. Chronobiol Int 16:377–414 El-Din El-Assal S, Alonso-Blanco C, Peeters AJ, Raz V, Koornneef M (2001) A QTL for flowering time in Arabidopsis reveals a novel allele of CRY2. Nat Genet 29:435–440 Fairchild CD, Schumaker MA, Quail PH (2000) HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev 14:2377–2391 Fankhauser C (2000) Phytochromes as light-modulated protein kinases. Semin Cell Dev Biol 11:467–473 Fankhauser C, Yeh KC, Lagarias JC, Zhang H, Elich TD, Chory J (1999) PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284:1539–1541 Folta KM, Spalding EP (2001a) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26:471–478 Folta KM, Spalding EP (2001b) Opposing roles of phytochrome A and phytochrome B in early cryptochrome-mediated growth inhibition. Plant J 28:333–340 Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J (1999) GIGANTEA: a circadian clockcontrolled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membranespanning domains. EMBO J 18:4679–4688 Garner WW, Allard HA (1920) Effects of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J Agric Res 18:223–606 Guo H, Yang H, Mockler TC, Lin C (1998) Regulation of flowering time by Arabidopsis photoreceptors. Science 279:1360– 1363 Guo H, Mockler T, Duong H, Lin C (2001) SUB1, an Arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science 291:487–490 Halliday KJ, Hudson M, Ni M, Qin M, Quail PH (1999) poc1: an Arabidopsis mutant perturbed in phytochrome signaling because of a T DNA insertion in the promoter of PIF3, a gene encoding a phytochrome-interacting bHLH protein. Proc Natl Acad Sci USA 96:5832–5837 Harmer SL, Hogenesch JB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA (2000) Orchestrated transcription of key pathways in arabidopsis by the circadian clock. Science 290:2110–2113 Harmer SL, Panda S, Kay SA (2001) Molecular bases of circadian rhythms. Annu Rev Cell Dev Biol 17:215–253 Harper RM, Stowe-Evans EL, Luesse DR, Muto H, Tatematsu K, Watahiki MK, Yamamoto K, Liscum E (2000) The NPH4 locus encodes the auxin response factor ARF7, a conditional regulator of differential growth in aerial Arabidopsis tissue. Plant Cell 12:757–770 Heintzen C, Nater M, Apel K, Staiger D (1997) AtGRP7, a nuclear RNA-binding protein as a component of a circadian-regulated negative feedback loop in Arabidopsis thaliana. Proc Natl Acad Sci USA 94:8515–8520 Heintzen C, Melzer S, Fischer R, Kappeler S, Apel K, Staiger D (1994a) A light- and temperature-entrained circadian clock controls expression of transcripts encoding nuclear proteins with homology to RNA-binding proteins in meristematic tissue. Plant J 5:799–813 Heintzen C, Fischer R, Melzer S, Kappeler K, Apel K, Staiger D (1994b) Circadian oscillations of a transcript encoding a germin-like protein that is associated with cell walls in young leaves of the long-day plant Sinapis alba L. Plant Physiol 106:905–915 Hicks KA, Millar AJ, Carre IA, Somers DE, Straume M, MeeksWagner DR, Kay SA (1996) Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science 274:790– 792 Hicks KA, Albertson TM, Wagner DR (2001) EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 13:1281–1292 Hudson ME (2000) The genetics of phytochrome signalling in Arabidopsis. Semin Cell Dev Biol 11:475–483 Huq E, Tepperman JM, Quail PH (2000) GIGANTEA is a nuclear protein involved in phytochrome signaling in Arabidopsis. Proc Natl Acad Sci USA 97:9789–9794 Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR (2001a) Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410:952–954 Jarillo JA, Capel J, Tang RH, Yang HQ, Alonso JM, Ecker JR, Cashmore AR (2001b) An Arabidopsis circadian clock component interacts with both CRY1 and phyB. Nature 410:487–490 Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291:2138–2141 Kendrick RE, Kronenberg G (1994) Photomorphogenesis in plants. Martinus Nijhoff, Dordrecht, Netherlands Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414:656–660 Kiyosue T, Wada M (2000) LKP1 (LOV kelch protein 1): a factor involved in the regulation of flowering time in arabidopsis. Plant J 23:807–815 Kleiner O, Kircher S, Harter K, Batschauer A (1999) Nuclear localization of the Arabidopsis blue light receptor cryptochrome 2. Plant J 19:289–296 Kloppstech K (1985) Diurnal and circadian rhythmicity in the expression of light-induced plan nuclear messenger RNAs. Planta 165:502–506 Kohchi T, Mukougawa K, Frankenberg N, Masuda M, Yokota A, Lagarias JC (2001) The Arabidopsis hy2 gene encodes phytochromobilin synthase, a ferredoxin-dependent biliverdin reductase. Plant Cell 13:425–436 Koornneef M, Rolff E, Spruit CJP (1980) Genetic control of lightinhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol [Suppl] 100:147–160 Kuno N, Furuya M (2000) Phytochrome regulation of nuclear gene expression in plants. Semin Cell Dev Biol 11:485–493 14 Lin C (2000a) Plant blue-light receptors. Trends Plant Sci 5:337– 342 Lin C (2000b) Photoreceptors and regulation of flowering time. Plant Physiol 123:39–50 Liu J, Yu J, McIntosh L, Kende H, Zeevaart JAD (2001) Isolation of a CONSTANS ortholog from Pharbitis nil and its role in flowering. Plant Physiol 125:1821–1830 Liu XL, Covington MF, Fankhauser C, Chory J, Wagner DR (2001) ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell 13:1293–1304 Ma L, Li J, Qu L, Hager J, Chen Z, Zhao H, Deng XW (2001) Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13:2589–2607 Mackenzie JM Jr., Coleman RA, Briggs WR, Pratt LH (1975) Reversible redistribution of phytochrome within the cell upon conversion to its physiologically active form. Proc Natl Acad Sci U S A 72:799–803 Makino S, Matsushika A, Kojima M, Yamashino T, Mizuno T (2002) The APRR1/TOC1 quintet implicated in circadian rhythms of Arabidopsis thaliana: I. Characterization with APRR1-overexpressing plants. Plant Cell Physiol 43:58–69 Maloof JN, Borevitz JO, Dabi T, Lutes J, Nehring RB, Redfern JL, Trainer GT, Wilson JM, Asami T, Berry CC, Weigel D, Chory J (2001) Natural variation in light sensitivity of Arabidopsis. Nat Genet 29:441–446 Martinez-Garcia JF, Huq E, Quail PH (2000) Direct targeting of light signals to a promoter element-bound transcription factor. Science 288:859–863 Mas P, Devlin PF, Panda S, Kay SA (2000) Functional interaction of phytochrome B and cryptochrome 2. Nature 408:207–211 Matsushika A, Makino S, Kojima M, Mizuno T (2000) Circadian waves of expression of the APRR1/TOC1 family of pseudoresponse regulators in Arabidopsis thaliana: insight into the plant circadian clock. Plant Cell Physiol 41:1002–1012 Matsushika A, Makino S, Kojima M, Yamashino T, Mizuno T (2002) The APRR1/TOC1 quintet implicated in circadian rhythms of Arabidopsis thaliana: II. Characterization with CCA1-overexpressing plants. Plant Cell Physiol 43:118–122 McClung CR (2001) Circadian rhythms in plants. Annu Rev Plant Physiol Plant Mol Biol 52:139–162 McDonald MJ, Rosbash M (2001) Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107:567–578 McWatters HG, Bastow RM, Hall A, Millar AJ (2000) The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature 408:716–720 McWatters HG, Roden LC, Staiger D (2001) Picking out parallels: plant circadian clocks in context. Philos Trans R Soc Lond B Biol Sci 356:1735–1743 Millar A, Straum MA, Chory J, Chua N-H, Kay S (1995a) Phytochrome and blue responsive photoreceptors regulate circadian period in Arabidopsis thaliana. Science 267:1163–1166 Millar A, Carré IA, Strayer C, Chua N-H, Kay S (1995b) Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 267:1161–1163 Millar AJ, Kay SA (1996) Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA 93:15491–15496 Moller SG, Kunkel T, Chua NH (2001) A plastidic ABC protein involved in intercompartmental communication of light signaling. Genes Dev 15:90–103 Mösinger E, Batschauer A, Vierstra RD, Apel K, Schäfer E (1987) Comparison of the effects of exogenous native phytochrome and in vivo irradiation on in vitro transcription in isolated nuclei form barley (Hordeum vulgare). Planta 170:505–514 Motchoulski A, Liscum E (1999) Arabidopsis NPH3: a NPH1 photoreceptor-interacting protein essential for phototropism. Science 286:961–964 Muramoto T, Kohchi T, Yokota A, Hwang I, Goodman HM (1999) The Arabidopsis photomorphogenic mutant hy1 is deficient in phytochrome chromophore biosynthesis as a result of a mutation in a plastid heme oxygenase. Plant Cell 11:335–348 Mustilli AC, Bowler C (1997) Tuning in to the signals controlling photoregulated gene expression in plants. EMBO J 16:5801– 5806 Nagy F, Schaefer E (2000) Nuclear and cytosolic events of lightinduced, phytochrome-regulated signaling in higher plants. EMBO J 19:157–163 Nagy F, Schaefer E (2002) Phytochromes control photomorphogenesis by differentially regulated, interacting signaling pathways in higher plants. Annu Rev Plant Physiol Plant Mol Biol 53:329–355 Nagy F, Kay SA, Chua N-H (1988) A circadian clock regulates transcription of the wheat Cab-1 gene. Genes Devel 2:376–382 N’Doye M, Millet B, Paynot M, Martin-Tanguy J (1994) Diurnal modulation of polyamine content in seedlings of Lycopersicon esculentum Mill. cv. Ace 55 by temperature and light. Plant Sci 104:11–15 Nelson DC, Lasswell J, Rogg LE, Cohen MA, Bartel B (2000) FKF1, a clock-controlled gene that regulates the transition to flowering in Arabidopsis. Cell 101:331–340 Ni M, Tepperman JM, Quail PH (1998) PIF3, a phytochromeinteracting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95:657–667 Ni M, Tepperman JM, Quail PH (1999) Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400:781–784 Osterlund MT, Ang LH, Deng XW (1999) The role of COP1 in repression of Arabidopsis photomorphogenetic development. Trends Cell Biol 9:113–118 Osterlund MT, Hardtke CS, Wei N, Deng XW (2000) Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405:462–466 Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh MS, Kim HJ, Kay SA, Nam HG (1999) Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285:1579–1582 Parks BM, Folta KM, Spalding EP (2001) Photocontrol of stem growth. Curr Opin Plant Biol 4:436–440 Quail PH (2002a) Photosensory perception and signalling in plant cells: new paradigms? Curr Opin Cell Biol 14:180–188 Quail PH (2002b) Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol 3:85–93 Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5:147–157 Reed JW, Nagpal P, Bastow RM, Solomon KS, Dowson-Day MJ, Elumalai RP, Millar AJ (2000) Independent action of ELF3 and phyB to control hypocotyl elongation and flowering time. Plant Physiol 122:1149–1160 Roden LC, Carré IA (2001) The molecular genetics of circadian rhythms in Arabidopsis. Semin Cell Dev Biol 12:305–315 Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2. A signal transducer of phototropic responses in Arabidopsis. Plant Cell 12:225–236 Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98:6969–6974 Sakamoto A, Nagatani A (1996) Nuclear localization activity of phytochrome B. Plant J 10:859–868 Salomon M, Eisenreich W, Durr H, Schleicher E, Knieb E, Massey V, Rudiger W, Muller F, Bacher A, Richter G (2001) An optomechanical transducer in the blue light receptor phototropin from Avena sativa. Proc Natl Acad Sci USA 98:12357–12361 Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G (2000) Distinct roles of 15 CONSTANS target genes in reproductive development of Arabidopsis. Science 288:1613–1616 Schaffer R, Ramsay N, Samach A C, S., Putterill J, Carré IA, Coupland G (1998) The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93:1219–1229 Schaffer R, Landgraf J, Accerbi M, Simon V, Larson M, Wisman E (2001) Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 13:113–123 Schultz TF, Kiyosue T, Yanovsky M, Wada M, Kay SA (2001) A role for LKP2 in the circadian clock of Arabidopsis. Plant Cell 13:2659–2670 Shinomura T, Uchida K, Furuya M (2000) Elementary processes of photoperception by phytochrome A for high irradiance response of hypocotyl elongation in Arabidopsis thaliana. Plant Physiol 122:147–156 Simpson GG, Dean C (2002) Arabidopsis, the rosetta stone of flowering time? Science 296:285–289 Smith H (2000) Phytochromes and light signal perception by plants – an emerging synthesis. Nature 407:585–591 Soh MS, Kim YM, Han SJ, Song PS (2000) REP1, a basic helixloop-helix protein, is required for a branch pathway of phytochrome A signaling in arabidopsis. Plant Cell 12:2061– 2074 Somers DE (1999) The physiology and molecular bases of the plant circadian clock. Plant Physiol 121:9–20 Somers DE, Devlin PF, Kay SA (1998a) Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 282:1488–1490 Somers DE, Webb AA, Pearson M, Kay SA (1998b) The shortperiod mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development 125:485–494 Somers DE, Schultz TF, Milnamow M, Kay SA (2000) ZEITLUPE encodes a novel clock-associated PAS protein from Arabidopsis. Cell 101:319–329 Spiegelman JI, Mindrinos MN, Fankhauser C, Richards D, Lutes J, Chory J, Oefner PJ (2000) Cloning of the Arabidopsis RSF1 gene by using a mapping strategy based on high-density DNA arrays and denaturing high-performance liquid chromatography. Plant Cell 12:2485–2498 Staiger D, Apel K (1999) Circadian clock-regulated expression of an RNA-binding protein in Arabidopsis: characterisation of a minimal promoter element. Mol Gen Genet 261:811–819 Staiger D, Heintzen C (1999) The circadian system of Arabidopsis thaliana: forward and reverse genetic approaches. Chronobiol Int 16:1–16. Staiger D, Apel K, Trepp G (1999) The Atger3 promoter confers circadian clock-regulated transcription with peak expression at the beginning of the night. Plant Mol Biol 40:873–882 Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC (1998) The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95:681–692 Stowe-Evans EL, Luesse DR, Liscum E (2001) The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via a photoreversible phytochrome A-dependent modulation of auxin responsiveness. Plant Physiol 126:826– 834 Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Mas P, Panda S, Kreps JA, Kay SA (2000) Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289:768–771 Suarez-Lopez P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G (2001) CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410:1116–1120 Sweere U, Luesse DR, Liscum E (2001) The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via photoreversible phytochrome A-dependent modulation of auxin responsiveness. Plant Physiol 126:826–834 Tepperman JM, Zhu T, Chang HS, Wang X, Quail PH (2001) Multiple transcription-factor genes are early targets of phytochrome A signaling. Proc Natl Acad Sci USA 98:9437–9442 Toth R, Kevei EE, Hall A, Millar AJ, Nagy F, Kozma-Bognar L (2001) Circadian clock-regulated expression of phytochrome and cryptochrome genes in Arabidopsis. Plant Physiol 127:1607–1616 Ueda HR, Matsumoto A, Kawamura M, Iino M, Tanimura T, Hashimoto S (2002) Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J Biol Chem 277:14048– 14052 van der Horst GTJ, Muitjtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker APM, van Leenen D, Buijis R, Bootsma D, Hoeijmakers JHJ, Yasui A (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398:627–630 Vierstra RD, Davis SJ (2000) Bacteriophytochromes: new tools for understanding phytochrome signal transduction. Semin Cell Dev Biol 11:511–521 Wagner E, Normann J, Albrechtova JTP, Walczysko P, Bonzon M, Greppin H (1998) Electrochemical-hydraulic signalling in photoperiodic control of flowering: is ‘‘florigen’’ a frequencycoded electrical signal? Flowering Newslett 26:62–74 Wang H, Deng XW (2002) Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J 21:1339–1349 Wang H, Ma LG, Li JM, Zhao HY, Deng XW (2001) Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294:154–158 Wang ZY, Tobin EM (1998) Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93:1207–1217 Whitelam GC, Devlin PF (1997) Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ 20:752– 758 Yang HQ, Wu YJ, Tang RH, Liu D, Liu Y, Cashmore AR (2000) The C termini of Arabidopsis cryptochromes mediate a constitutive light response. Cell 103:815–827. Yang HQ, Tang RH, Cashmore AR (2001) The signaling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13:2573–2587 Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12:2473– 2484 Yanovsky MJ, Kay SA (2001) Signaling networks in the plant circadian system. Curr Opin Plant Biol 4:429–435 Yanovsky MJ, Mazzella MA, Casal JJ (2000a) A quadruple photoreceptor mutant still keeps track of time. Curr Biol 10:1013– 1015 Yanovsky MJ, Whitelam GC, Casal JJ (2000b) fhy3-1 retains inductive responses of phytochrome A. Plant Physiol 123:235– 242 Yanovsky MJ, Mazzella MA, Whitelam GC, Casal JJ (2001) Resetting of the circadian clock by phytochromes and cryptochromes in Arabidopsis. J Biol Rhythms 16:523–530 Yeh K, Wu S, Murphy J, Lagarias JC (1997) A cyanobacterial phytochrome two-component light sensory system. Science 277:1505–1508 Yeh KC, Lagarias JC (1998) Eukaryotic phytochromes: light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc Natl Acad Sci USA 95:13976–13981 Young MW, Kay SA (2001) Time zones: a comparative genetics of circadian clocks. Nat Rev Genet 2:702–715 Zhong HH, Resnick AS, Straume M, McClung CR (1997) Effects of synergistic signaling by phytochrome A and cryptochrome 1 on circadian clock-regulated catalase expression. Plant Cell 9: 947–955 16 Zhong HH, Painter JE, Salome PA, Straume M, McClung CR (1998) Imbibition, but not release from stratification, sets the circadian clock in Arabidopsis seedlings. Plant Cell 10:2005– 2017 Zhou YC, Dieterle M, Buche C, Kretsch T (2002) The negatively acting factors EID1 and SPA1 have distinct functions in phytochrome A-specific light signaling. Plant Physiol 128:1098– 1108 Zhu Y, Tepperman JM, Fairchild CD, Quail PH (2000) Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc Natl Acad Sci USA 97:13419–13424